Cr3+ in Phyllosilicates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky
Kentucky Geological Survey James C. Cobb, State Geologist and Director University of Kentucky, Lexington A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky John D. Calandra Thesis Series 2 Series XII, 2000 Kentucky Geological Survey James C. Cobb, State Geologist and Director University of Kentucky, Lexington A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky John D. Calandra On the cover: Photomicrographs of olivine phenoc- rysts: (top) a stressed first-generation olivine pheno- cryst and (bottom) a late-stage olivine phenocryst. Thesis Series 2 Series XII, 2000 i UNIVERSITY OF KENTUCKY Computer and Laboratory Services Section: Charles T. Wethington Jr., President Steven Cordiviola, Head Fitzgerald Bramwell, Vice President for Research and Richard E. Sergeant, Geologist IV Graduate Studies Joseph B. Dixon, Information Technology Manager I Jack Supplee, Director, Administrative Affairs, Research James M. McElhone, Information Systems Technical and Graduate Studies Support Specialist IV Henry E. Francis, Scientist II KENTUCKY GEOLOGICAL SURVEY ADVISORY Karen Cisler, Scientist I BOARD Jason S. Backus, Research Analyst Henry M. Morgan, Chair, Utica Steven R. Mock, Research Analyst Ron D. Gilkerson, Vice Chair, Lexington Tracy Sizemore, Research Analyst William W. Bowdy, Fort Thomas Steve Cawood, Frankfort GEOLOGICAL DIVISION Hugh B. Gabbard, Winchester Coal and Minerals Section: Kenneth Gibson, Madisonville Donald R. Chesnut Jr., Head Mark E. Gormley, Versailles Garland R. Dever Jr., Geologist V Rosanne Kruzich, Louisville Cortland F. Eble, Geologist V W.A. Mossbarger, Lexington Gerald A. Weisenfluh, Geologist V Jacqueline Swigart, Louisville David A. Williams, Geologist V, Henderson office John F. Tate, Bonnyman Stephen F. Greb, Geologist IV David A. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Iron.Rich Amesite from the Lake Asbestos Mine. Black
Canodian Mineralogist Yol.22, pp. 43742 (1984) IRON.RICHAMESITE FROM THE LAKE ASBESTOS MINE. BLACKLAKE. OUEBEC MEHMET YEYZT TANER,* AND ROGER LAURENT DAporternentde Gdologie,Universitd Loval, Qudbec,Qudbec GIK 7P4 ABSTRACT o 90.02(1l)', P W.42(12)',1 89.96(8)'.A notreconnais- sance,c'est la premibrefois qu'on ddcritune am6site riche Iron-rich amesite is found in a metasomatically altered enfer. Elles'ct form€ependant l'altdration hydrothermale granite sheet20 to 40 cm thick emplacedin serpentinite of du granitedans la serpentinite,dans les m€mes conditions the Thetford Mi[es ophiolite complex at the Lake Asbestos debasses pression et temperaturequi ont prdsid6d la for- mine (z16o01'N,11"22' W) ntheQuebec Appalachians.The mation de la rodingite dansle granite et de I'amiante- amesiteis associatedsdth 4lodingife 6semblage(grossu- chrysotiledans la serpentinite. lar + calcite t diopside t clinozoisite) that has replaced the primary minerals of the granite. The Quebec amesite Mots-clds:am6site, rodingite, granite, complexeophio- occurs as subhedral grains 2@ to 6@ pm.in diameter that litique, Thetford Mines, Qu6bec. have a tabular habit. It is optically positive with a small 2V, a 1.612,1 1.630,(t -'o = 0.018).Its structuralfor- INTRoDUc"iloN mula, calculated from electron-microprobe data, is: (Mg1.1Fe6.eA1s.e)(Alo.esil.df Os(OH)r.2. X-ray powder- Amesite is a raxehydrated aluminosilicate of mag- diffraction yield data dvalues that are systematicallygreater nesium in which some ferrous iron usually is found than those of amesitefrom Chester, Massachusetts,prob- replacingmapesium. The extent of this replacement ably becauseof the partial replacement of Mg by Fe. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

06 11 09 Separation & Preparation of Biotite and Muscovite Samples For
06 11 09 Separation & Preparation of Biotite and Muscovite samples for 40Ar/39Ar analysis Karl Lang When dealing with detrital samples, sample contamination is a big problem. To avoid this at all costs be clean, this means handling samples one at a time and cleaning all equipment entirely after each sample handling. Only handle samples in a quite (i.e. not windy) environment, where there is little chance of spilling or blowing samples away. Handle samples over clean copier paper, and change the paper after each sample. Use compressed air (either canned or from a compressor) to clean all equipment in separation stages and methanol to keep equipment dirt and dust free in the preparation stages. This can be often be tedious work, I recommend finding a good book on tape or podcast to listen to. Estimate times to completion are stated. Detrital Samples Separation I. Sample drying (1-3 days) For detrital samples it is likely that samples will be wet. Simple let the samples dry in the open air, or under a mild lamp on paper plates. It may be necessary to occasionally stir up samples to get them to dry faster, if you do this, clean the stirrer after each sample. Split samples using a riffle splitter to obtain a quantity for the rest of the process, do this in the rock room. II. Sample Sieving (3-7 days) First locate a set of sieves that can be intensively cleaned. There are a set of appropriate sieves in 317 for this use, be careful using other's sieves as you will likely bend the meshes during cleaning. -

Muscovite Solid Solutions in the System K20-Mgo-Feo-A1203-Sio2-H20: an Experimental Study at 2 Kbar Ph2o and Comparison with Natural Li-Free White Micas
MINERALOGICAL MAGAZINE, JUNE 1986, VOL. 50, PP. 257-66 Muscovite solid solutions in the system K20-MgO-FeO-A1203-SiO2-H20: an experimental study at 2 kbar PH2o and comparison with natural Li-free white micas GILLES MoNIER Laboratoire de P&rologie, Universit6 d'Or16ans, 45046 Orlrans Cedex, France AND JI~AN-LouIs ROBERT Centre de Recherche sur la Synthrse et Chimie des MinSraux, G.I.S.C.N.R.S.-B.R.G.M., 1A rue de la Frrollerie, 45071 Odrans Cedex 2, France ABSTRACT. This paper presents the results of an experi- K EY W OR O S : muscovite, phengite, solid solution, crystal- mental study of muscovite solid solutions in the system chemistry, experimental mineralogy, granites, hydro- K20-M~+O-A1203 SiO2-H20 (HF), with M2+= thermal alteration. Mg 2+ or Fe 2§ in the temperature range 300 700~ under 2 kbar P.~o, Muscovite solid solutions can be described, in this system, as the result of two substitutions. NATURAL lithium-free white micas are generally One is the phengitic substitution (x), which preserves the described as solid solutions between the muscovite pure dioctahedral character of the mica; the second is the end member K(A12R)(Si3AI)Olo(OH)2, where [] biotitic substitution (y), which leads to trioctahedral stands for an octahedral vacant site, and the micas and does not change the composition of the celadonite end member K(AIM z+ [3)Si4010(OH)2, tetrahedral layer Si3AI. The general formula of muscovite with M 2 + = Mg 2., Fe E+, thus, they are considered in this system is K(A12_~_2y/aM2+yOl_y/a)(Sia+~All_x) to belong to the so-called phengitic series. -

Fingerprints of Kamafugite-Like Magmas in Mesozoic Lamproites of the Aldan Shield: Evidence from Olivine and Olivine-Hosted Inclusions
minerals Article Fingerprints of Kamafugite-Like Magmas in Mesozoic Lamproites of the Aldan Shield: Evidence from Olivine and Olivine-Hosted Inclusions Ivan F. Chayka 1,2,*, Alexander V. Sobolev 3,4, Andrey E. Izokh 1,5, Valentina G. Batanova 3, Stepan P. Krasheninnikov 4 , Maria V. Chervyakovskaya 6, Alkiviadis Kontonikas-Charos 7, Anton V. Kutyrev 8 , Boris M. Lobastov 9 and Vasiliy S. Chervyakovskiy 6 1 V. S. Sobolev Institute of Geology and Mineralogy Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia; [email protected] 2 Institute of Experimental Mineralogy, Russian Academy of Sciences, 142432 Chernogolovka, Russia 3 Institut des Sciences de la Terre (ISTerre), Université de Grenoble Alpes, 38041 Grenoble, France; [email protected] (A.V.S.); [email protected] (V.G.B.) 4 Vernadsky Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, Russia; [email protected] 5 Department of Geology and Geophysics, Novosibirsk State University, 630090 Novosibirsk, Russia 6 Institute of Geology and Geochemistry, Ural Branch of the Russian Academy of Sciences, 620016 Yekaterinburg, Russia; [email protected] (M.V.C.); [email protected] (V.S.C.) 7 School of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide, SA 5005, Australia; [email protected] 8 Institute of Volcanology and Seismology, Far Eastern Branch of the Russian Academy of Sciences, 683000 Petropavlovsk-Kamchatsky, Russia; [email protected] 9 Institute of Mining, Geology and Geotechnology, Siberian Federal University, 660041 Krasnoyarsk, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-985-799-4936 Received: 17 February 2020; Accepted: 6 April 2020; Published: 9 April 2020 Abstract: Mesozoic (125–135 Ma) cratonic low-Ti lamproites from the northern part of the Aldan Shield do not conform to typical classification schemes of ultrapotassic anorogenic rocks. -
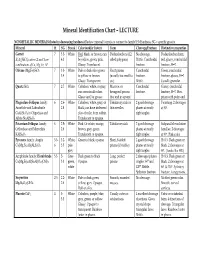
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Cation Ordering and Pseudosymmetry in Layer Silicates'
I A merican M ineralogist, Volume60. pages175-187, 1975 Cation Ordering and Pseudosymmetryin Layer Silicates' S. W. BerI-nv Departmentof Geologyand Geophysics,Uniuersity of Wisconsin-Madison Madison, Wisconsin5 3706 Abstract The particular sequenceof sheetsand layers present in the structure of a layer silicate createsan ideal symmetry that is usually basedon the assumptionsof trioctahedralcompositions, no significantdistor- tion, and no cation ordering.Ordering oftetrahedral cations,asjudged by mean l-O bond lengths,has been found within the constraints of the ideal spacegroup for specimensof muscovite-3I, phengile-2M2, la-4 Cr-chlorite, and vermiculite of the 2-layer s type. Many ideal spacegroups do not allow ordering of tetrahedralcations because all tetrahedramust be equivalentby symmetry.This includesthe common lM micasand chlorites.Ordering oftetrahedral cations within subgroupsymmetries has not beensought very often, but has been reported for anandite-2Or, llb-2prochlorite, and Ia-2 donbassite. Ordering ofoctahedral cations within the ideal spacegroups is more common and has been found for muscovite-37, lepidolite-2M", clintonite-lM, fluoropolylithionite-lM,la-4 Cr-chlorite, lb-odd ripidolite, and vermiculite. Ordering in subgroup symmetries has been reported l-oranandite-2or, IIb-2 prochlorite, and llb-4 corundophilite. Ordering in local out-of-step domains has been documented by study of diffuse non-Bragg scattering for the octahedral catlons in polylithionite according to a two-dimensional pattern and for the interlayer cations in vermiculite over a three-cellsuperlattice. All dioctahedral layer silicates have ordered vacant octahedral sites, and the locations of the vacancies change the symmetry from that of the ideal spacegroup in kaolinite, dickite, nacrite, and la-2 donbassite Four new structural determinations are reported for margarite-2M,, amesile-2Hr,cronstedtite-2H", and a two-layercookeite. -

Phyllosilicates in the Sediment-Forming Processes: Weathering, Erosion, Transportation, and Deposition
Acta Geodyn. Geomater., Vol. 6, No. 1 (153), 13–43, 2009 PHYLLOSILICATES IN THE SEDIMENT-FORMING PROCESSES: WEATHERING, EROSION, TRANSPORTATION, AND DEPOSITION Jiří KONTA Faculty of Sciences, Charles University, Albertov 6, 128 43 Prague 2 Home address: Korunní 127, 130 00 Prague 3, Czech Republic *Corresponding author‘s e-mail: [email protected] (Received October 2008, accepted January 2009) ABSTRACT Phyllosilicates are classified into the following groups: 1 - Neutral 1:1 structures: the kaolinite and serpentine group. 2 - Neutral 2:1 structures: the pyrophyllite and talc group. 3 - High-charge 2:1 structures, non-expansible in polar liquids: illite and the dioctahedral and trioctahedral micas, also brittle micas. 4 - Low- to medium-charge 2:1 structures, expansible phyllosilicates in polar liquids: smectites and vermiculites. 5 - Neutral 2:1:1 structures: chlorites. 6 - Neutral to weak-charge ribbon structures, so-called pseudophyllosilicates or hormites: palygorskite and sepiolite (fibrous crystalline clay minerals). 7 - Amorphous clay minerals. Order-disorder states, polymorphism, polytypism, and interstratifications of phyllosilicates are influenced by several factors: 1) a chemical micromilieu acting during the crystallization in any environment, including the space of clay pseudomorphs after original rock-forming silicates or volcanic glasses; 2) the accepted thermal energy; 3) the permeability. The composition and properties of parent rocks and minerals in the weathering crusts, the elevation, and topography of source areas and climatic conditions control the intensity of weathering, erosion, and the resulting assemblage of phyllosilicates to be transported after erosion. The enormously high accumulation of phyllosilicates in the sedimentary lithosphere is primarily conditioned by their high up to extremely high chemical stability in water-rich environments (expressed by index of corrosion, IKO).