Chondritic Magnesium Isotopic Composition of the Terrestrial Mantle: a Case Study of Peridotite Xenoliths from the North China Craton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky
Kentucky Geological Survey James C. Cobb, State Geologist and Director University of Kentucky, Lexington A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky John D. Calandra Thesis Series 2 Series XII, 2000 Kentucky Geological Survey James C. Cobb, State Geologist and Director University of Kentucky, Lexington A Ground Magnetic Survey of Kimberlite Intrusives in Elliott County, Kentucky John D. Calandra On the cover: Photomicrographs of olivine phenoc- rysts: (top) a stressed first-generation olivine pheno- cryst and (bottom) a late-stage olivine phenocryst. Thesis Series 2 Series XII, 2000 i UNIVERSITY OF KENTUCKY Computer and Laboratory Services Section: Charles T. Wethington Jr., President Steven Cordiviola, Head Fitzgerald Bramwell, Vice President for Research and Richard E. Sergeant, Geologist IV Graduate Studies Joseph B. Dixon, Information Technology Manager I Jack Supplee, Director, Administrative Affairs, Research James M. McElhone, Information Systems Technical and Graduate Studies Support Specialist IV Henry E. Francis, Scientist II KENTUCKY GEOLOGICAL SURVEY ADVISORY Karen Cisler, Scientist I BOARD Jason S. Backus, Research Analyst Henry M. Morgan, Chair, Utica Steven R. Mock, Research Analyst Ron D. Gilkerson, Vice Chair, Lexington Tracy Sizemore, Research Analyst William W. Bowdy, Fort Thomas Steve Cawood, Frankfort GEOLOGICAL DIVISION Hugh B. Gabbard, Winchester Coal and Minerals Section: Kenneth Gibson, Madisonville Donald R. Chesnut Jr., Head Mark E. Gormley, Versailles Garland R. Dever Jr., Geologist V Rosanne Kruzich, Louisville Cortland F. Eble, Geologist V W.A. Mossbarger, Lexington Gerald A. Weisenfluh, Geologist V Jacqueline Swigart, Louisville David A. Williams, Geologist V, Henderson office John F. Tate, Bonnyman Stephen F. Greb, Geologist IV David A. -

Fingerprints of Kamafugite-Like Magmas in Mesozoic Lamproites of the Aldan Shield: Evidence from Olivine and Olivine-Hosted Inclusions
minerals Article Fingerprints of Kamafugite-Like Magmas in Mesozoic Lamproites of the Aldan Shield: Evidence from Olivine and Olivine-Hosted Inclusions Ivan F. Chayka 1,2,*, Alexander V. Sobolev 3,4, Andrey E. Izokh 1,5, Valentina G. Batanova 3, Stepan P. Krasheninnikov 4 , Maria V. Chervyakovskaya 6, Alkiviadis Kontonikas-Charos 7, Anton V. Kutyrev 8 , Boris M. Lobastov 9 and Vasiliy S. Chervyakovskiy 6 1 V. S. Sobolev Institute of Geology and Mineralogy Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia; [email protected] 2 Institute of Experimental Mineralogy, Russian Academy of Sciences, 142432 Chernogolovka, Russia 3 Institut des Sciences de la Terre (ISTerre), Université de Grenoble Alpes, 38041 Grenoble, France; [email protected] (A.V.S.); [email protected] (V.G.B.) 4 Vernadsky Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, Russia; [email protected] 5 Department of Geology and Geophysics, Novosibirsk State University, 630090 Novosibirsk, Russia 6 Institute of Geology and Geochemistry, Ural Branch of the Russian Academy of Sciences, 620016 Yekaterinburg, Russia; [email protected] (M.V.C.); [email protected] (V.S.C.) 7 School of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide, SA 5005, Australia; [email protected] 8 Institute of Volcanology and Seismology, Far Eastern Branch of the Russian Academy of Sciences, 683000 Petropavlovsk-Kamchatsky, Russia; [email protected] 9 Institute of Mining, Geology and Geotechnology, Siberian Federal University, 660041 Krasnoyarsk, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-985-799-4936 Received: 17 February 2020; Accepted: 6 April 2020; Published: 9 April 2020 Abstract: Mesozoic (125–135 Ma) cratonic low-Ti lamproites from the northern part of the Aldan Shield do not conform to typical classification schemes of ultrapotassic anorogenic rocks. -

Cr3+ in Phyllosilicates
Mineral Spectroscopy: A Tribute to Roger G. Bums © The Geochemical Society, Special Publication No.5, ]996 Editors: M. D. Dyar, C. McCammon and M. W. Schaefer 3 Cr + in phyllosilicates: Influence of the nature of coordinating ligands and their next cationic neighbors on the crystal field parameters I 2 2 A. N. PLATONOV , K. LANGER , M. ANDRUT .3, G. CALAS4 'Institute of Geochemistry, Mineralogy and Ore Formation, Academy of Science of Ukraine, 252680 Kiev, Ukraine 2Institute of Mineralogy and Crystallography, Technical University, D-10623 Berlin, Germany 3GeoForschungszentrum Potsdam, D-14473 Potsdam, Deutschland "Laboratoire de Mineralogie et de Cristallographie, Universite de Paris 6 et 7, F-7525l Paris, France 3 Abstract- The electronic absorption spectra of Cr + -bearing clinochlore (I, kammererite), amesite (II), muscovite (III, fuchsite), dickite (IV), and montmorillonite (V, volkonskite) analysed by electron microprobe were obtained on single crystals. Microscope-spectrometric techniques and polarized radiation in the spectral range 10000-38000 cm " (I, II, III) or (on fine grained material) diffuse reflectance spectrometry in the spectral range 8000-50000 cm-I (IV, V) were used. The ligand field theoretical evaluation of the spectra showed the following: (i) The fl.o = 10Dq = f(1/R5) relation, wherein fl.o is the octahedral crystal field parameter and R the mean cation ligand distance, is valid within each series of layer silicates containing octahedral Cr3+ either in a trioctahedral layer (I, II and phlogopite) or in a dioctahedral layer (III, IV, V). Between the two functions, fl.o.trioct = f(1lR~ioct) and fl.o.di=t = f(1/R~ioct), there exists an energy difference of about 2200 em -I. -

On the Mechanism of Exfoliation of 'Vermiculite'
Clay Minerals, (2013) 48, 563–582 OPEN ACCESS On the mechanism of exfoliation of ‘Vermiculite’ 1,2, 3,4 3 S. HILLIER *, E. M. M. MARWA AND C. M. RICE 1 The James Hutton Institute, Craigiebuckler, Aberdeen, AB15 8QH, UK, 2 Department of Soil and Environment, Swedish University of Agricultural Sciences (SLU), P.O. Box 7014, SE-750 07 Uppsala, Sweden, 3 Department of Geology and Petroleum Geology, University of Aberdeen, AB24 3UE, UK, and 4 Sokoine University of Agriculture, Department of Soil Science, P. O. Box 3008, Morogoro, Tanzania (Received 20 May 2013; revised 27 July 2013; Editor: George Christidis) ABSTRACT: Six samples of ‘Vermiculite’ have been studied to investigate the mechanism of its well known but poorly understood property to exfoliate. The samples were analysed quantitatively by XRD to determine their precise mineralogical composition. Electron microprobe methods, including elemental mapping of native potassium and of caesium (introduced by cation exchange) were used to examine variation in the chemical composition of the particles. Most of the samples examined show heterogeneous mineralogical compositions which occur as distinct zones within the volume of individual particles, presenting a mosaic texture. Exfoliation is related to this mosaic distribution of the different mineral phases within the particles. Lateral phase boundaries between vermiculite and mica layers, or vermiculite and chlorite layers are postulated to prevent or impede the escape of gas from a particle, resulting in exfoliation when the pressure exceeds the interlayer bonding forces that hold the layers together. This mechanism provides a common explanation for the exfoliation of ‘Vermiculite’ by thermal methods or by treatment with H2O2. -

Identification Tables for Common Minerals in Thin Section
Identification Tables for Common Minerals in Thin Section These tables provide a concise summary of the properties of a range of common minerals. Within the tables, minerals are arranged by colour so as to help with identification. If a mineral commonly has a range of colours, it will appear once for each colour. To identify an unknown mineral, start by answering the following questions: (1) What colour is the mineral? (2) What is the relief of the mineral? (3) Do you think you are looking at an igneous, metamorphic or sedimentary rock? Go to the chart, and scan the properties. Within each colour group, minerals are arranged in order of increasing refractive index (which more or less corresponds to relief). This should at once limit you to only a few minerals. By looking at the chart, see which properties might help you distinguish between the possibilities. Then, look at the mineral again, and check these further details. Notes: (i) Name: names listed here may be strict mineral names (e.g., andalusite), or group names (e.g., chlorite), or distinctive variety names (e.g., titanian augite). These tables contain a personal selection of some of the more common minerals. Remember that there are nearly 4000 minerals, although 95% of these are rare or very rare. The minerals in here probably make up 95% of medium and coarse-grained rocks in the crust. (ii) IMS: this gives a simple assessment of whether the mineral is common in igneous (I), metamorphic (M) or sedimentary (S) rocks. These are not infallible guides - in particular many igneous and metamorphic minerals can occur occasionally in sediments. -

Clay Minerals
CLAY MINERALS CD. Barton United States Department of Agriculture Forest Service, Aiken, South Carolina, U.S.A. A.D. Karathanasis University of Kentucky, Lexington, Kentucky, U.S.A. INTRODUCTION of soil minerals is understandable. Notwithstanding, the prevalence of silicon and oxygen in the phyllosilicate structure is logical. The SiC>4 tetrahedron is the foundation Clay minerals refers to a group of hydrous aluminosili- 2 of all silicate structures. It consists of four O ~~ ions at the cates that predominate the clay-sized (<2 |xm) fraction of apices of a regular tetrahedron coordinated to one Si4+ at soils. These minerals are similar in chemical and structural the center (Fig. 1). An interlocking array of these composition to the primary minerals that originate from tetrahedral connected at three corners in the same plane the Earth's crust; however, transformations in the by shared oxygen anions forms a hexagonal network geometric arrangement of atoms and ions within their called the tetrahedral sheet (2). When external ions bond to structures occur due to weathering. Primary minerals form the tetrahedral sheet they are coordinated to one hydroxyl at elevated temperatures and pressures, and are usually and two oxygen anion groups. An aluminum, magnesium, derived from igneous or metamorphic rocks. Inside the or iron ion typically serves as the coordinating cation and Earth these minerals are relatively stable, but transform- is surrounded by six oxygen atoms or hydroxyl groups ations may occur once exposed to the ambient conditions resulting in an eight-sided building block termed an of the Earth's surface. Although some of the most resistant octohedron (Fig. -

Download PDF About Minerals Sorted by Mineral Group
MINERALS SORTED BY MINERAL GROUP Most minerals are chemically classified as native elements, sulfides, sulfates, oxides, silicates, carbonates, phosphates, halides, nitrates, tungstates, molybdates, arsenates, or vanadates. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). NATIVE ELEMENTS (DIAMOND, SULFUR, GOLD) Native elements are minerals composed of only one element, such as copper, sulfur, gold, silver, and diamond. They are not common in Kentucky, but are mentioned because of their appeal to collectors. DIAMOND Crystal system: isometric. Cleavage: perfect octahedral. Color: colorless, pale shades of yellow, orange, or blue. Hardness: 10. Specific gravity: 3.5. Uses: jewelry, saws, polishing equipment. Diamond, the hardest of any naturally formed mineral, is also highly refractive, causing light to be split into a spectrum of colors commonly called play of colors. Because of its high specific gravity, it is easily concentrated in alluvial gravels, where it can be mined. This is one of the main mining methods used in South Africa, where most of the world's diamonds originate. The source rock of diamonds is the igneous rock kimberlite, also referred to as diamond pipe. A nongem variety of diamond is called bort. Kentucky has kimberlites in Elliott County in eastern Kentucky and Crittenden and Livingston Counties in western Kentucky, but no diamonds have ever been discovered in or authenticated from these rocks. A diamond was found in Adair County, but it was determined to have been brought in from somewhere else. SULFUR Crystal system: orthorhombic. Fracture: uneven. Color: yellow. Hardness 1 to 2. -

Master's Thesis
MASTER'S THESIS Mineral Chemistry of Gangue Minerals in the Kiirunavaara Iron Ore Joakim Nordstrand Master of Science (120 credits) Exploration and Environmental Geosciences Luleå University of Technology Department of Civil, Environmental and Natural resources engineering Fall 08 Till T. Abstract The iron-ore of Kiirunavaara is one of the largest magnetite-apatite mineralisation in the world. It is well known for its purity and size and its origin has been the subject of an intense debate for over a century. The composition of the most important minerals from an economic point of view, magnetite and apatite, has been well studied but little is known about the composition and role of the silicate minerals. Nowadays, even the silicates have been targeted for investigations, both in Kiirunavaara and in similar deposits elsewhere, e.g., Sierra la Banderra, Chile. The growing interest derives mainly from the importance of the gangue minerals in ore processing but also because they might hold the key to the origin of the ores. In this study, a large scale microprobe investigation of over 250 analyses has been carried out mainly focussing on silicates within the ore body but also to some extent on sulphates, phosphates and carbonates. Most of the analysed grains belonged to either the amphibole group or the mica group. Amphiboles and micas are by far the most abundant silicates within the ore body. Through (EMPA) analysis of 198 separate mineral grains, this investigation led to the broadest and most updated characterisation of silicates within the Kiirunavaara ore body. It also briefly looks at setting and chemistry of sulphates, phosphates, and carbonates. -
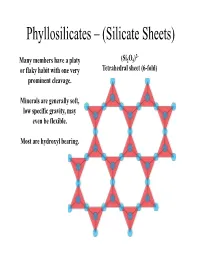
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups. -

DIAMOND-BEARING PERIDOTITE in PIKE COUNTY, ARKANSAS. by HUGH D. MISEK and CLARENCE S. Ross. Peridotite Is Exposed in Four Places
DIAMOND-BEARING PERIDOTITE IN PIKE COUNTY, ARKANSAS. By HUGH D. MISEK and CLARENCE S. Ross. LOCATION OF THE AREA. Peridotite is exposed in four places near Murfreesboro, Pike County, in southwestern Arkansas, and at three of these places diamonds have been obtained. (See fig. 65.) The exposure that Scale 23 SO Miles Nephelitfi syenite and related igneous rocks FIOUKE 65. Map of parts of Arkansas, Oklahoma, and adjacent States, showing the area (shaded rec tangle) that contains the diamond-bearing peridotite of Pike County, Ark. was first discovered, which has been known to geologists since 1842, lies 2£ miles south-southeast of Murfreesboro, near the con fluence of Prairie Creek with Little Missouri River, and is here called the Prairie Creek area. (See PL VIII.) Diamonds were discovered in this area in 1906, and several thousand of them, ranging in weight from a small fraction of a carat to 20£ carats, have since been produced. The mines in this area are the Ozark, 109930° 23 19 279 280 CONTRIBUTIONS TO ECONOMIC GEOLOGY, 1922, PART I. Mauney, and Arkansas. All the other exposures of peridotite occur within an area of 1 square mile about 2 miles northeast of the Prairie Creek area and 3 miles S. 75° E. of Murfreesboro. Two of these exposures are here named the Kimberlite and American areas, from the Kimberlite and American mines, which are in them; they are in sec. 14, T. 8 S., R. 25 W. A third exposure, known as the Black Lick area, is at and near the Black Lick, in the northwest corner of sec. -

Hydrophobic Surface State of Talc and How It Is
HYDROPHOBIC SURFACE STATE OF TALC AND HOW IT IS INFLUENCED BY POLYSACCHARIDE ADSORPTION by Venkata Veeren Babu Atluri A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Metallurgical Engineering The University of Utah May 2019 Copyright © Venkata Veeren Babu Atluri 2019 All Rights Reserved The University of Utah Graduate School STATEMENT OF DISSERTATION APPROVAL The dissertation of Venkata Veeren Babu Atluri has been approved by the following supervisory committee members: Jan D Miller , Chair 01/15/2019 Date Approved Xuming Wang , Member 01/15/2019 Date Approved Michael L. Free , Member 01/15/2019 Date Approved Vladimir Hlady , Member 01/15/2019 Date Approved York R. Smith , Member 01/15/2019 Date Approved and by Michael Simpson , Chair/Dean of the Department/College/School of Metallurgical Engineering and by David B. Kieda, Dean of The Graduate School. ABSTRACT Talc is both an important industrial mineral product recovered by flotation, and also in other cases, a gangue mineral of concern in the flotation of certain sulfide ores, such as the PGM ores in South Africa and in the United States. The talc face surface is naturally hydrophobic with a contact angle of nearly 80º, which accounts for its flotation recovery in one case, and its contamination of sulfide mineral concentrates in other instances. High- quality talc structures were investigated using surface analysis techniques including contact angle analysis, high-speed video bubble attachment measurements, atomic force microscopy, molecular dynamics simulation (MDS), microflotation, and film thickness measurements by Synchronized Triwavelength Reflection Interferometry Microscopy (STRIM). -

Nomenclature of the Micas
Mineralogical Magazine, April 1999, Vol. 63(2), pp. 267-279 Nomenclature of the micas M. RIEDER (CHAIRMAN) Department of Geochemistry, Mineralogy and Mineral Resources, Charles University, Albertov 6, 12843 Praha 2, Czech Republic G. CAVAZZINI Dipartimento di Mineralogia e Petrologia, Universith di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy Yu. S. D'YAKONOV VSEGEI, Srednii pr., 74, 199 026 Sankt-Peterburg, Russia W. m. FRANK-KAMENETSKII* G. GOTTARDIt S. GUGGENHEIM Department of Geological Sciences, University of Illinois at Chicago, 845 West Taylor St., Chicago, IL 60607-7059, USA P. V. KOVAL' Institut geokhimii SO AN Rossii, ul. Favorskogo la, Irkutsk - 33, Russia 664 033 G. MOLLER Institut fiir Mineralogie und Mineralische Rohstoffe, Technische Universit/it Clausthal, Postfach 1253, D-38670 Clausthal-Zellerfeld, Germany A. M, R. NEIVA Departamento de Ci6ncias da Terra, Universidade de Coimbra, Apartado 3014, 3049 Coimbra CODEX, Portugal E. W. RADOSLOVICH$ J.-L. ROBERT Centre de Recherche sur la Synth6se et la Chimie des Min6raux, C.N.R.S., 1A, Rue de la F6rollerie, 45071 Od6ans CEDEX 2, France F. P. SASSI Dipartimento di Mineralogia e Petrologia, Universit~t di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy H. TAKEDA Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino City, Chiba 275, Japan Z. WEISS Central Analytical Laboratory, Technical University of Mining and Metallurgy, T/'. 17.1istopadu, 708 33 Ostrava- Poruba, Czech Republic AND D. R. WONESw * Russia; died 1994 t Italy; died 1988 * Australia; resigned 1986 wUSA; died 1984 1999 The Mineralogical Society M. RIEDER ETAL. ABSTRACT I I End-members and species defined with permissible ranges of composition are presented for the true micas, the brittle micas, and the interlayer-deficient micas.