Ferri-Annite from the Dales Gorge Member Iron-Formations, Wittenoom Area, Western Australia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chemical and Structural Evolution of “Metamorphic Vermiculite” in Metaclastic Rocks of the Betic Cordillera, Málaga, Spain: a Synthesis
249 The Canadian Mineralogist Vol. 44, pp. 249-265 (2006) CHEMICAL AND STRUCTURAL EVOLUTION OF “METAMORPHIC VERMICULITE” IN METACLASTIC ROCKS OF THE BETIC CORDILLERA, MÁLAGA, SPAIN: A SYNTHESIS MARÍA DOLORES RUIZ CRUZ§ Departamento de Química Inorgánica, Cristalografía y Mineralogía, Universidad de Málaga, Campus de Teatinos, E-29071 Málaga, Spain JOSÉ MIGUEL NIETO Departamento de Geología, Facultad de Ciencias Experimentales, Universidad de Huelva, E-21071 Huelva, Spain ABSTRACT Vermiculite-like minerals from a metamorphic sequence of the Betic Cordillera, near Málaga, southeastern Spain, were investigated in detail by X-ray diffraction, electron-microprobe analysis, and transmission-analytical electron microscopy. Our results reveal that the chlorite-to-biotite transformation is much more complex than previously assumed. In addition to mixed- layer minerals with a mica:chlorite ratio of 2:1 and 1:1, which had previously been identifi ed, mixed-layer phases with very high and very low chlorite:vermiculite ratios have been identifi ed, together with true vermiculite, as intermediate steps in the chlorite-to-biotite transformation. The observed sequence is: chlorite → random to ordered 1:2 mica–chlorite mixed-layer phases → regular 1:1 chlorite–vermiculite mixed-layer phases → Vrm-rich chlorite–vermiculite mixed-layer phases → vermiculite → biotite. This sequence includes a continuous increase of interlayer cation content, and is similar to that described in the smectite- to-illite transformation. The high Na content of most of these phases suggests that in the absence of K-feldspar, two parallel sites of reactants, chlorite + phengite and chlorite + albite, account for the formation of vermiculitic phases, and later, of biotite. Keywords: mixed-layer minerals, metamorphic vermiculite, X-ray diffraction, electron-microprobe analysis, transmission elec- tron microscopy, analytical electron microscopy, Betic Cordillera, Spain. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

The Structure of Stilpnomelane Reexamined
THE STRUCTURE OF STILPNOMELANE REEXAMINED JouN W. Gnunon, Uniaersity of Minnesota, Mi.nneapolis,Minnesota. Assrnect New r-ray data show the distribution of ions normal to the basal cleavage in the layer silicate stilpnomelane. Since it is similarto talc and biotite a structure consistentwithits properties can be proposed. It explains satisfactorily the behavior of the mineral including its base exchange of K for Tl. Stilpnomelane is an important essential constituent of certain iron formations. fNrnooucrroN The writer (1) made an attempt in 1937 to determine the composition and crystal structure of stilpnomelane. At that time the mineral had been reported from the quartz veins in iron formations and from the chlorite-epidote-albite schists in New Zealand (8 and 11). Recently it has been identified in large amounts in the iron formations of the Cuyuna and Mesabi ranges of Minnesota. There it is one of the three principal iron silicates,iron talc, to be describedin detail shortly, being the second and greenalite the third. As was pointed out previously, (1, p. 912) stilpnomelane may readily be mistaken for biotite under the microscope.Like biotite it is negative with a small optic angle and has similar pleochroism. In the hand speci- men it resembleseither biotite or chlorite, but is much more brittle. Its cleavageis excellent. Basal sections with partially developed hexagonal outlines have been observed. These properties and the discussion that follows make it certain that stilpnomelane has a layer structure and is related to the micas and chlorites. CnBurcar CouposrrroN Nothing essentially new can be added with regard to the chemical composition of stilpnomelane. -

Muscovite Solid Solutions in the System K20-Mgo-Feo-A1203-Sio2-H20: an Experimental Study at 2 Kbar Ph2o and Comparison with Natural Li-Free White Micas
MINERALOGICAL MAGAZINE, JUNE 1986, VOL. 50, PP. 257-66 Muscovite solid solutions in the system K20-MgO-FeO-A1203-SiO2-H20: an experimental study at 2 kbar PH2o and comparison with natural Li-free white micas GILLES MoNIER Laboratoire de P&rologie, Universit6 d'Or16ans, 45046 Orlrans Cedex, France AND JI~AN-LouIs ROBERT Centre de Recherche sur la Synthrse et Chimie des MinSraux, G.I.S.C.N.R.S.-B.R.G.M., 1A rue de la Frrollerie, 45071 Odrans Cedex 2, France ABSTRACT. This paper presents the results of an experi- K EY W OR O S : muscovite, phengite, solid solution, crystal- mental study of muscovite solid solutions in the system chemistry, experimental mineralogy, granites, hydro- K20-M~+O-A1203 SiO2-H20 (HF), with M2+= thermal alteration. Mg 2+ or Fe 2§ in the temperature range 300 700~ under 2 kbar P.~o, Muscovite solid solutions can be described, in this system, as the result of two substitutions. NATURAL lithium-free white micas are generally One is the phengitic substitution (x), which preserves the described as solid solutions between the muscovite pure dioctahedral character of the mica; the second is the end member K(A12R)(Si3AI)Olo(OH)2, where [] biotitic substitution (y), which leads to trioctahedral stands for an octahedral vacant site, and the micas and does not change the composition of the celadonite end member K(AIM z+ [3)Si4010(OH)2, tetrahedral layer Si3AI. The general formula of muscovite with M 2 + = Mg 2., Fe E+, thus, they are considered in this system is K(A12_~_2y/aM2+yOl_y/a)(Sia+~All_x) to belong to the so-called phengitic series. -

Approaches to the Low Grade Metamorphic History of the Karakaya Complex by Chlorite Mineralogy and Geochemistry
Minerals 2015, 5, 221-246; doi:10.3390/min5020221 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Approaches to the Low Grade Metamorphic History of the Karakaya Complex by Chlorite Mineralogy and Geochemistry Sema Tetiker 1, Hüseyin Yalçın 2,* and Ömer Bozkaya 3 1 Department of Geological Engineering, Batman University, 72100 Batman, Turkey; E-Mail: [email protected] 2 Department of Geological Engineering, Cumhuriyet University, 58140 Sivas, Turkey 3 Department of Geological Engineering, Pamukkale University, 20070 Denizli, Turkey; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +90-0542-412-16-19. Academic Editor: Antonio Simonetti Received: 18 November 2014 / Accepted: 9 April 2015 / Published: 16 April 2015 Abstract: In this study, chlorite is used to investigate the diagenetic-metamorphic evolution and accurate geological history of the different units belonging to the Karakaya complex, Turkey. Primary and secondary chlorite minerals in the very low-grade metamorphic rocks display interference colors of blue and brown and an appearance of optical isotropy. Chlorites are present in the matrix, pores, and/or rocks units as platy/flaky and partly radial forms. X-ray diffraction (XRD) data indicate that Mg-Fe chlorites with entirely IIb polytype (trioctahedral) exhibit a variety of compositions, such as brunsvigite-diabantite-chamosite. The major element contents and structural formulas of chlorite also suggest these were derived from both felsic and metabasic source rocks. Trace and rare earth element (REE) concentrations of chlorites increase with increasing grade of metamorphism, and these geochemical changes can be related to the tectonic structures, formational mechanics, and environments present during their generation. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Stilpnomelane K(Fe2+,Mg,Fe3+)
2+ 3+ Stilpnomelane K(Fe ; Mg; Fe )8(Si; Al)12(O; OH)27 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Triclinic. Point Group: 1: As plates or scales and ¯bers with comb structures; as plumose or radiating groups, to 1 cm; as a velvety coating. Physical Properties: Cleavage: Perfect on 001 , imperfect on 010 . Hardness = 3{4 D(meas.) = 2.59{2.96 D(calc.) = 2.667 f g f g Optical Properties: Semitransparent. Color: Black, greenish black, yellowish bronze, greenish bronze; in thin section, golden brown, dark brown, green. Luster: Pearly to vitreous on cleavage surface, may be submetallic. Optical Class: Biaxial ({). Pleochroism: X = bright golden yellow to pale yellow; Y = Z = deep reddish brown to deep green to nearly black. Orientation: Y = b; X (001). ® = 1.543{1.634 ¯ = 1.576{1.745 ° = 1.576{1.745 2V(meas.) = 0 ' ? » ± Cell Data: Space Group: P 1: a = 21.86{22.05 b = 21.86{22.05 c = 17.62{17.74 ® = 124:14 125:65 ¯ = 95:86 95:93 ° = 120:00 Z = 6 ±¡ ± ±¡ ± ± X-ray Powder Pattern: Crystal Falls, Iron Co., Michigan, USA. 12.3 (100), 4.16 (100), 2.55 (100), 2.69 (70), 3.12 (60), 1.568 (60), 6.26 (50) Chemistry: (1) (2) (1) (2) SiO2 44.45 48.03 MgO 2.77 4.94 TiO2 0.23 CaO 0.53 0.83 Al2O3 7.26 6.48 Na2O 0.03 Fe2O3 20.82 4.12 K2O 2.06 0.83 + FeO 14.04 22.88 H2O 6.41 6.90 MnO 0.05 2.67 H2O¡ 1.35 2.64 Total 99.77 100.55 (1) Zuckmantel, Poland. -

Miissbauer Spectral Study of Ferruginous One-Layer Trioctahedral
American Mineralogist, Volume 71, pages 955-965, 1986 Miissbauer spectral study of ferruginousone-layer trioctahedral micas M. DAnsv DYAn,* Rocnn G. BunNs Department of Earth, Atmospheric, and Planetary Sciences,Massachusetts Institute of Technology, Cambridge, Massachusetts02 I 39 Ansrn-r.cr A Mdssbauerspectral study is describedof I 5 potassicfemrginous I M micas representing a wide rangeof chemical compositions and parageneses.Considerable precaution was taken to alleviate problems due to oxidation and preferred orientation of mica platelets during sample preparation. Biotite Mdssbauer spectrawere resolved into two Fe2+and two Fe3+ doublets assignedto cations in the two cis-M2 and one trans-Ml positions. Deviations of peak areasfrom the site multiplicity 2:l ratio demonstratedthat Fe2+ions are relatively enriched in cis-M2 positions and that this is accentuatedin biotites from metamorphic rocks. Tetrahedral Fe3+ions were confirmed in ferriphlogopite, ferriannites from banded iron formations, and Someannites. Annite from the type locality in Massachusettscontains high concentrations of octahedral Fe2+and Fe3+cations in comparable amounts, in ac- cordancewith Dana's (1868, ,4 System of Mineralogy, 5th edition) original definition of this annite. Of the samplesstudied, the annite from Pikes Peak, Colorado, approximates most closely Winchell's (1925, American Journal of Science,5th ser., 9, 309-327) classi- fication of annite as the Fe2+endmember analogueof phlogopite. IurnooucrroN when the bulk compositions contain adequate(Si + Al) Iron-bearing -

Known Minerals
Recognition and Description of Minerals - Knowns ISOTROPIC MINERALS Halite NaCl Sylvite KCl Fluorite CaF2 Periclase MgO 2+ 3+ Garnet (Ca,Mg,Fe ,Mn)3Al,Fe ,Cr)2(SiO4)3 ERSC 2P22 – Brock University Greg Finn Recognition and Description of Minerals - Knowns UNIAXIAL MINERALS Quartz SiO2 Calcite CaCO3 Nepheline NaAlSiO4 Apatite Ca5(PO4)3(F,Cl,OH) Zircon ZrSiO4 Tourmaline Boro-Silicate ERSC 2P22 – Brock University Greg Finn Recognition and Description of Minerals - Knowns BIAXIAL MINERALS Olivine (Mg,Fe)2SiO4 Orthopyroxene (Mg,Fe)2Si2O6 Clinopyroxene (Ca,Mg,Fe,Al)2Si2O6 Hornblende Ca2(Mg,Fe,Al)5Si8O22(OH)2 Tremolite Ca2Mg5Si8O22(OH)2 Actinolite Ca2Fe5Si8O22(OH)2 Biotite K2(Mg,Fe)3AlSi3O10(OH,F)2 Muscovite KAl2(AlSi3O10)(OH)2 Chlorite (Mg,Al,Fe)3(Si,Al)4O10(OH)2*(Mg,Al,Fe)3(OH)6 Plagioclase (Na,Ca)Al(Al,Si)Si2O8 Alkali Feldspar KAlSi3O8 ERSC 2P22 – Brock University Greg Finn 1 Features Useful in the Identification of a Mineral • Shape of grains • Degree of crystallinity • Cleavage • Twinning • Alteration • Associations ERSC 2P22 – Brock University Greg Finn Shape of grains • Acicular needle-like grains (actinolite, tremolite) • Bladed elongate, slender (hornblende) • Columnar elongate with equidimensional cross sections (quartz, pyroxenes) • Equant equidimensional grains (quartz, olivine) • Fibrous grains form long slender fibers (asbestos, sillimanite) • Lathlike flat elongate grains (plagioclase) • Prismatic crystal faces defined by prism (apatite) • Tabular book shape (plagioclase) ERSC 2P22 – Brock University Greg Finn Bladed Grains Hornblende © G.C. Finn, 2005 ERSC 2P22 – Brock University Greg Finn 2 Equant Grains qtz qtz © G.C. Finn, 2005 © G.C. Finn, 2005 ol ol © G.C. -

Initiation and Evolution of Subduction: T-T-D History of the Easton Metamorphic Suite, Northwest Washington State
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Fall 2017 Initiation and Evolution of Subduction: T-t-D History of the Easton Metamorphic Suite, Northwest Washington State Jeremy Cordova Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Cordova, Jeremy, "Initiation and Evolution of Subduction: T-t-D History of the Easton Metamorphic Suite, Northwest Washington State" (2017). WWU Graduate School Collection. 626. https://cedar.wwu.edu/wwuet/626 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. INITIATION AND EVOLUTION OF SUBDUCTION: T-t-D HISTORY OF THE EASTON METAMORPHIC SUITE, NORTHWEST WASHINGTON STATE By Jeremy Cordova Accepted in Partial Completion of the Requirements for the Degree Master of Science in Geology ADVISORY COMMITTEE Co-Chair, Dr. Elizabeth Schermer Co-Chair, Dr. Sean Mulcahy Dr. Susan DeBari GRADUATE SCHOOL Dr. Gautam Pillay, Dean Master’s Thesis In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non- exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. -
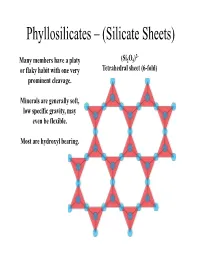
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups.