Index to Volume 85, 1973
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of the Rallidae
A CLASSIFICATION OF THE RALLIDAE STARRY L. OLSON HE family Rallidae, containing over 150 living or recently extinct species T and having one of the widest distributions of any family of terrestrial vertebrates, has, in proportion to its size and interest, received less study than perhaps any other major group of birds. The only two attempts at a classifi- cation of all of the recent rallid genera are those of Sharpe (1894) and Peters (1934). Although each of these lists has some merit, neither is satisfactory in reflecting relationships between the genera and both often separate closely related groups. In the past, no attempt has been made to identify the more primitive members of the Rallidae or to illuminate evolutionary trends in the family. Lists almost invariably begin with the genus Rdus which is actually one of the most specialized genera of the family and does not represent an ancestral or primitive stock. One of the difficulties of rallid taxonomy arises from the relative homo- geneity of the family, rails for the most part being rather generalized birds with few groups having morphological modifications that clearly define them. As a consequence, particularly well-marked genera have been elevated to subfamily rank on the basis of characters that in more diverse families would not be considered as significant. Another weakness of former classifications of the family arose from what Mayr (194933) referred to as the “instability of the morphology of rails.” This “instability of morphology,” while seeming to belie what I have just said about homogeneity, refers only to the characteristics associated with flightlessness-a condition that appears with great regularity in island rails and which has evolved many times. -

A Trait Dataset for Taiwan's Breeding Birds
Biodiversity Data Journal 8: e49735 doi: 10.3897/BDJ.8.e49735 Data Paper A trait dataset for Taiwan's breeding birds Pei-Yu Tsai‡, Chie-Jen Ko §,|, Chia Hsieh¶#, Yi-Ting Su , Ya-Jung Lu‡, Ruey-Shing Lin§, Mao-Ning Tuanmu¤ ‡ Biodiversity Research Center, Academia Sinica, Taipei, Taiwan § Endemic Species Research Institute, Jiji, Nantou, Taiwan | Institute of Ecology and Evolutionary Biology, National Taiwan University, Taipei, Taiwan ¶ BioSciences Department, Rice University, Houston, United States of America # Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan ¤ Thematic Center for Systematics and Biodiversity Informatics, Biodiversity Research Center, Academia Sinica, Taipei, Taiwan Corresponding author: Mao-Ning Tuanmu ([email protected]) Academic editor: Cynthia Parr Received: 30 Dec 2019 | Accepted: 08 May 2020 | Published: 19 May 2020 Citation: Tsai P-Y, Ko C-J, Hsieh C, Su Y-T, Lu Y-J, Lin R-S, Tuanmu M-N (2020) A trait dataset for Taiwan's breeding birds. Biodiversity Data Journal 8: e49735. https://doi.org/10.3897/BDJ.8.e49735 Abstract Background Species traits affect how a species interacts with the environment and other species and thus determine the role of the species in an ecosystem. They affect not only population dynamics of a species across space and over time, but also community structure and function through their key role in the community assembly processes. Information on species traits is also highly relevant for conservation planning as they determine the adaptive ability of a species in the face of environmental changes. However, information on species traits is usually scarce and sparsely distributed amongst diverse types of literature and sources. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
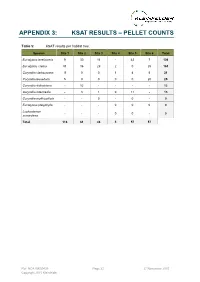
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

Supplementary Methods S1
1 Validation methods for trophic niche models 2 3 To assign links between nodes (species), we used trophic niche-space models (e.g., [1]). 4 Each of these models has two quantile regressions that define the prey-size range a 5 predator of a given size is predicted to consume. Species whose body mass is within the 6 range of a predator’s prey size, as identified by the trophic niche-space model, are predicted 7 to be prey, while those outside the range are predicted not to be eaten. 8 9 The broad taxonomy of a predator helps to predict predation interactions [2]. To optimize 10 our trophic niche-space model, we therefore tested whether including taxonomic class of 11 predators improved the fit of quantile regressions. Using trophic (to identify which species 12 were predators), body mass, and taxonomic data, we fitted and compared five quantile 13 regression models (including a null model) to the GloBI data. In each model, we log10- 14 transformed the dependent variable prey body mass, and included for the independent 15 variables different combinations of log10-transformed predator body mass, predator class, 16 and the interaction between these variables (Supplementary Table S4). We log10- 17 transformed both predator and prey body mass to linearize the relationship between these 18 variables. We fit the five quantile regressions to the upper and lower 5% of prey body mass, 19 and compared model fits using the Bayesian information criterion (BIC). The predator body 20 mass*predator class model fit the 95th quantile data best, whereas the predator body mass 21 + predator class model fit the 5th quantile data marginally better than the aforementioned 22 interaction model (Supplementary Figure S2, Supplementary Table S4). -

Standards for Aquatic/Semi-Aquatic Bird Sanctuaries
Global Federation of Animal Sanctuaries Standards For Aquatic/Semi-Aquatic Bird Sanctuaries Version: April 2019 ©2012 Global Federation of Animal Sanctuaries Global Federation of Animal Sanctuaries – Standards for Aquatic/Semi-Aquatic Bird Sanctuaries Table of Contents INTRODUCTION ............................................................................................................ 1 GFAS PRINCIPLES ............................................................................................................................................................. 1 ANIMALS COVERED BY THESE STANDARDS ................................................................................................. 1 AQUATIC/SEMI-AQUATIC BIRD STANDARDS ............................................................................................. 3 AQUATIC/SEMI-AQUATIC BIRD HOUSING ....................................................... 3 H-1. Types of Space and Size .................................................................................................................................................... 3 H-2. Containment ................................................................................................................................................................................ 5 H-3. Ground and Plantings ........................................................................................................................................................... 6 H-4. Gates and Doors ...................................................................................................................................................................... -

Supporting References for Nelson & Ellis
Supplemental Data for Nelson & Ellis (2018) The citations below were used to create Figures 1 & 2 in Nelson, G., & Ellis, S. (2018). The History and Impact of Digitization and Digital Data Mobilization on Biodiversity Research. Publication title by year, author (at least one ADBC funded author or not), and data portal used. This list includes papers that cite the ADBC program, iDigBio, TCNs/PENs, or any of the data portals that received ADBC funds at some point. Publications were coded as "referencing" ADBC if the authors did not use portal data or resources; it includes publications where data was deposited or archived in the portal as well as those that mention ADBC initiatives. Scroll to the bottom of the document for a key regarding authors (e.g., TCNs) and portals. Citation Year Author Portal used Portal or ADBC Program was referenced, but data from the portal not used Acevedo-Charry, O. A., & Coral-Jaramillo, B. (2017). Annotations on the 2017 Other Vertnet; distribution of Doliornis remseni (Cotingidae ) and Buthraupis macaulaylibrary wetmorei (Thraupidae ). Colombian Ornithology, 16, eNB04-1 http://asociacioncolombianadeornitologia.org/wp- content/uploads/2017/11/1412.pdf [Accessed 4 Apr. 2018] Adams, A. J., Pessier, A. P., & Briggs, C. J. (2017). Rapid extirpation of a 2017 Other VertNet North American frog coincides with an increase in fungal pathogen prevalence: Historical analysis and implications for reintroduction. Ecology and Evolution, 7, (23), 10216-10232. Adams, R. P. (2017). Multiple evidences of past evolution are hidden in 2017 Other SEINet nrDNA of Juniperus arizonica and J. coahuilensis populations in the trans-Pecos, Texas region. -

Diversity and Endemism in Tidal-Marsh Vertebrates
Studies in Avian Biology No. 32:32-53 DIVERSITY AND ENDEMISM IN TIDAL-MARSH VERTEBRATES RUSSELL GREENBERG AND JESúS E. MALDONADO Abstract. Tidal marshes are distributed patcliily, predominantly along the mid- to high-latitude coasts of the major continents. The greatest extensions of non-arctic tidal marshes are found along the Atlantic and Gulf coasts of North America, but local concentrations can be found in Great Britain, northern Europe, northern Japan, northern China, and northern Korea, Argentina-Uruguay-Brazil, Australia, and New Zealand. We tallied the number of terrestrial vertebrate species that regularly occupy tidal marshes in each of these regions, as well as species or subspecies that are largely restricted to tidal marshes. In each of the major coastal areas we found 8-21 species of breeding birds and 13-25 species of terrestrial mammals. The diversity of tidal-marsh birds and mammals is highly inter-correlated, as is the diversity of species restricted to saltmarshes. These values are, in turn, correlated with tidal-marsh area along a coastline. We estimate approximately seven species of turtles occur in brackish or saltmarshes worldwide, but only one species is endemic and it is found in eastern North America. A large number of frogs and snakes occur opportunistically in tidal marshes, primarily in southeastern United States, particularly Florida. Three endemic snake taxa are restricted to tidal marshes of eastern North America as well. Overall, only in North America were we able to find documentation for multiple taxa of terrestrial vertebrates associated with tidal marshes. These include one species of mammal and two species of birds, one species of snake, and one species of turtle. -

Habitat Selection and Calling Activity of the Lewin's Rail (Lewinia
Queensland University of Technology School of Earth, Environmental and Biological Sciences Habitat Selection and Calling Activity of the Lewin’s Rail (Lewinia pectoralis pectoralis) Jennifer Gibson (B.App.Sc.) 2017 A thesis submitted in fulfilment of the requirements for the degree of Masters of Applied Science at the Queensland University of Technology Key Words Lewin’s Rail, Lewinia pectoralis pectoralis, grassland, habitat selection, vegetation structure, call playback, calling activity, acoustic sensor, remote sensing, fire response. Abstract The Lewin’s Rail, a Near Threatened cryptic ground dwelling bird, inhabits wetlands containing dense emergent or fringing vegetation. The populations are disjunct and exist between Kangaroo Island (SA) and Townsville (Qld) and all around the Southern and Eastern coast of mainland Australia. It is a poorly studied bird and little is known about its ecology, however literature suggests that vegetation structure is the key element to Lewin’s Rail habitat use. In recognition of upcoming major construction in and around known Lewin’s Rail habitat at Brisbane Airport, Brisbane Airport Corporation (BAC) wanted to minimise the impact of construction and where possible, maintain habitat integrity. This thesis is result of the research commission by them to identify the Rail habitat features and minimise disturbance. Extensive vegetation surveys and bird surveys were carried out between April 2007 and November 2008. Of the three types of Lewin’s Rail calls “kek kek’, “squeaky door” and “grunt” the “kek kek” is understood to be the territorial call. The birds are very cryptic so this call was used for call playback in order to establish presence/absence at 90 different locations within 15 defined sites across the BAC study area. -

Schnaitman, Rachel.Pdf
A REVIEW OF THREATS TO ISLAND ENDEMIC RAILS by Rachel Schnaitman A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Bachelor of Science in Wildlife Conservation with Distinction. Spring 2010 Copyright 2010 Rachel Schnaitman All Rights Reserved A REVIEW OF THREATS TO ISLAND ENDEMIC RAILS by Rachel Schnaitman Approved:______________________________________________________________ W.Gregory Shriver, Ph.D. Professor in charge of the thesis on behalf of the Advisory Committee Approved:______________________________________________________________ Jacob L. Bowman, Ph.D. Committee member from the Department of Entomology and Wildlife Ecology Approved: _____________________________________________________________ K. Kniel, Ph.D. Committee member from the Board of Senior Thesis Readers Approved:_____________________________________________________________ Ismat Shah, Ph.D. Chair of the University Committee on Student and Faculty Honors ACKNOWLEDGMENTS I would like to thank the Undergraduate Research Program for allowing me to be involved with the research program. I would also like to thank my advisor, Dr. Greg Shriver for supporting me and guiding me through this process. Furthermore I would like to thank my committee, Dr. J. Bowman and Dr. K. Kniel. iii TABLE OF CONTENTS LIST OF TABLES ....................................................................................................... vi LIST OF FIGURES ................................................................................................... -

Full Account (PDF)
FULL ACCOUNT FOR: Felis catus Felis catus System: Terrestrial Kingdom Phylum Class Order Family Animalia Chordata Mammalia Carnivora Felidae Common name cat (English), domestic cat (English), pusiniveikau (English, Fiji), house cat (English), Hauskatze (German), poti (Maori), feral cat (English) Synonym Similar species Summary Felis catus was domesticated in the eastern Mediterranean c. 3000 years ago. Considering the extent to which cats are valued as pets, it is not surprising that they have since been translocated by humans to almost all parts of the world. Notable predators, cats threaten native birdlife and other fauna, especially on islands where native species have evolved in relative isolation from predators. view this species on IUCN Red List Species Description Felis catus is a small animal in the wild (up to 5kg, but more commonly 1.5 -3.0kg) but may be considerably heavier when domesticated. Colour is extremely variable in domesticated varieties and feral cats commonly revert to black, tabby or tortoiseshell with varying extents of white starting from the belly and breast. Lifecycle Stages Gestation: 65 days. Weaning: 35-40 days. Sexual maturity: 9 months. Global Invasive Species Database (GISD) 2021. Species profile Felis catus. Available Pag. 1 from: http://www.iucngisd.org/gisd/species.php?sc=24 [Accessed 07 October 2021] FULL ACCOUNT FOR: Felis catus Habitat Description Feral cats adapt to a variety of habitat types and circumstances. On the Australian continent they inhabit forests and woodland habitats in eastern, western and northern parts of the country (Dickman 1996). On Hahajima Island, Japan, feral cats have been observed widely in various kinds of habitats, including primary forests (Kawakami and Higuchi 2002). -

Are Domestic Cats Exterminating Wildlife?
Are Domestic Cats Exterminating Wildlife? „thecutout‟ All-free-download.com By Ben Isacat August 2016 It is asserted that cats are exterminating species and should be vigorously controlled. What truth is there to this allegation? ? Nature has made domestic cats superbly repeated so often that many people accept it adapted predators of small animals, like as fact: rodents and birds. Predators, being at the top of the food web, are normally rare; they cannot “Domestic cats have tremendous be more numerous than the prey they feed on impacts on wildlife and are or they would die off. However, domestic cats responsible for the extinction of are unlike any other mammal predator numerous mammals, reptiles, and at (domestic dogs excepted): humans have made least 33 bird species globally.” The cats super-abundant and spread them all over Wildlife Society (2011) the globe. Domestic cats are now counted in their millions. The claim that domestic cats exterminate species drums up public hate for cats. Given that domestic cats are such passionate Professional and supposedly objective and abundant predators, scientists are researchers are not immune to ailurophobia. exploring their predatory behaviour to Nico Dauphine, a bird researcher who worked understand how they affect wildlife. This has at the National Zoo, Washington DC, led some researchers to claim that cats are announced at a bird conference: exterminating species. It is important to understand this claim because many people “Historically, cats have been are now demanding that cats are eliminated or specifically implicated in at least 33 severely controlled. So what is the basis for bird extinctions, making them one of this claim? the most important causes of bird extinctions worldwide” And added that, Do Cats Exterminate Species? “…feral animal removal should A much publicised and often repeated become a permanent, regular feature assertion about domestic cats is that “Feral of wildlife management.” (Dauphine et cats are responsible for the extinction of at al 2009). -

Rails, Gallinules and Coots Subfamily RALLINAE Rafinesque
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 177-179. Order GRUIFORMES: Rails, Cranes and Allies Family RALLIDAE Rafinesque: Rails, Gallinules and Coots This classification and nomenclature of rails largely follows Taylor & van Perlo (1998). Subfamily RALLINAE Rafinesque: Rails, Gallinules and Coots Rallia Rafinesque, 1815: Analyse de la Nature: 70 – Type genus Rallus Linnaeus, 1758. Genus Lewinia G.R. Gray Lewinia G.R. Gray, 1855: Cat. Genera Subgen. Birds Brit. Mus.: 120 – Type species (by monotypy) Rallus lewinii Swainson = Lewinia pectoralis (Temminck). Donacias Heine & Reichenow, 1890: Nom. Mus. Hein. Ornith.: 321. Unnecessary nomen novum for Lewinia G.R. Gray, 1855. Hyporallus Iredale & Mathews, 1926: Bull. Brit. Ornith. Club 46: 76 – Type species (by original designation) Rallus muelleri Rothschild = Lewinia muelleri (Rothschild). Lewinia muelleri (Rothschild) Auckland Island Rail Rallus muelleri Rothschild, 1893: Bull. Brit. Ornith. Club 1(8): 40 – Auckland Island. Hypotaenidia muelleri (Rothschild); Buller 1905, Suppl. Birds N.Z. 1: 42. Rallus pectoralis muelleri Rothschild; Checklist Committee 1953, Checklist N.Z. Birds: 39. Rallus brachipus von Hugel 1875; Oliver 1955, New Zealand Birds, 2nd edition: 351. Not Rallus brachipus Swainson, 1838. Lewinia muelleri (Rothschild); Sibley & Monroe 1990, Distr. and Taxon. Birds of the World: 225. Dryolimnas pectoralis muelleri (Rothschild); Marchant & Higgins 1993, HANZAB 2: 529. Dryolimnas muelleri (Rothschild); Holdaway et al.