Reconstructing the Ups and Downs of Primate Brain Evolution: Implications for Adaptive Hypotheses and Homo Floresiensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

8. Primate Evolution
8. Primate Evolution Jonathan M. G. Perry, Ph.D., The Johns Hopkins University School of Medicine Stephanie L. Canington, B.A., The Johns Hopkins University School of Medicine Learning Objectives • Understand the major trends in primate evolution from the origin of primates to the origin of our own species • Learn about primate adaptations and how they characterize major primate groups • Discuss the kinds of evidence that anthropologists use to find out how extinct primates are related to each other and to living primates • Recognize how the changing geography and climate of Earth have influenced where and when primates have thrived or gone extinct The first fifty million years of primate evolution was a series of adaptive radiations leading to the diversification of the earliest lemurs, monkeys, and apes. The primate story begins in the canopy and understory of conifer-dominated forests, with our small, furtive ancestors subsisting at night, beneath the notice of day-active dinosaurs. From the archaic plesiadapiforms (archaic primates) to the earliest groups of true primates (euprimates), the origin of our own order is characterized by the struggle for new food sources and microhabitats in the arboreal setting. Climate change forced major extinctions as the northern continents became increasingly dry, cold, and seasonal and as tropical rainforests gave way to deciduous forests, woodlands, and eventually grasslands. Lemurs, lorises, and tarsiers—once diverse groups containing many species—became rare, except for lemurs in Madagascar where there were no anthropoid competitors and perhaps few predators. Meanwhile, anthropoids (monkeys and apes) emerged in the Old World, then dispersed across parts of the northern hemisphere, Africa, and ultimately South America. -

Haplogroup Relationships Between Domestic and Wild Sheep Resolved Using a Mitogenome Panel
Heredity (2011) 106, 700–706 & 2011 Macmillan Publishers Limited All rights reserved 0018-067X/11 www.nature.com/hdy ORIGINAL ARTICLE Haplogroup relationships between domestic and wild sheep resolved using a mitogenome panel JRS Meadows1,3, S Hiendleder2 and JW Kijas1 1CSIRO Livestock Industries, St Lucia, Queensland, Australia and 2School of Agriculture, Food and Wine & Research Centre for Reproductive Health, School of Animal and Veterinary Science, University of Adelaide, Roseworthy, South Australia, Australia Five haplogroups have been identified in domestic sheep 920 000±190 000 years ago based on protein coding through global surveys of mitochondrial (mt) sequence sequence. The utility of various mtDNA components to inform variation, however these group classifications are often the true relationship between sheep was also examined with based on small fragments of the complete mtDNA sequence; Bayesian, maximum likelihood and partitioned Bremmer sup- partial control region or the cytochrome B gene. This study port analyses. The control region was found to be the mtDNA presents the complete mitogenome from representatives of component, which contributed the highest amount of support to each haplogroup identified in domestic sheep, plus a sample the tree generated using the complete data set. This study of their wild relatives. Comparison of the sequence success- provides the nucleus of a mtDNA mitogenome panel, which can fully resolved the relationships between each haplogroup be used to assess additional mitogenomes and serve as a and provided insight into the relationship with wild sheep. reference set to evaluate small fragments of the mtDNA. The five haplogroups were characterised as branching Heredity (2011) 106, 700–706; doi:10.1038/hdy.2010.122; independently, a radiation that shared a common ancestor published online 13 October 2010 Keywords: Ovis aries; domestication; mitochondria; genome; diversity Introduction Pedrosa et al., 2005; Pereira et al., 2006; Tapio et al., 2006; Meadows et al., 2007). -

Fossil Primates
AccessScience from McGraw-Hill Education Page 1 of 16 www.accessscience.com Fossil primates Contributed by: Eric Delson Publication year: 2014 Extinct members of the order of mammals to which humans belong. All current classifications divide the living primates into two major groups (suborders): the Strepsirhini or “lower” primates (lemurs, lorises, and bushbabies) and the Haplorhini or “higher” primates [tarsiers and anthropoids (New and Old World monkeys, greater and lesser apes, and humans)]. Some fossil groups (omomyiforms and adapiforms) can be placed with or near these two extant groupings; however, there is contention whether the Plesiadapiformes represent the earliest relatives of primates and are best placed within the order (as here) or outside it. See also: FOSSIL; MAMMALIA; PHYLOGENY; PHYSICAL ANTHROPOLOGY; PRIMATES. Vast evidence suggests that the order Primates is a monophyletic group, that is, the primates have a common genetic origin. Although several peculiarities of the primate bauplan (body plan) appear to be inherited from an inferred common ancestor, it seems that the order as a whole is characterized by showing a variety of parallel adaptations in different groups to a predominantly arboreal lifestyle, including anatomical and behavioral complexes related to improved grasping and manipulative capacities, a variety of locomotor styles, and enlargement of the higher centers of the brain. Among the extant primates, the lower primates more closely resemble forms that evolved relatively early in the history of the order, whereas the higher primates represent a group that evolved more recently (Fig. 1). A classification of the primates, as accepted here, appears above. Early primates The earliest primates are placed in their own semiorder, Plesiadapiformes (as contrasted with the semiorder Euprimates for all living forms), because they have no direct evolutionary links with, and bear few adaptive resemblances to, any group of living primates. -

Diversity and Evolution of the Mhc-DRB1 Gene in the Two Endemic Iberian Subspecies of Pyrenean Chamois, Rupicapra Pyrenaica
Heredity (2007) 99, 406–413 & 2007 Nature Publishing Group All rights reserved 0018-067X/07 $30.00 www.nature.com/hdy ORIGINAL ARTICLE Diversity and evolution of the Mhc-DRB1 gene in the two endemic Iberian subspecies of Pyrenean chamois, Rupicapra pyrenaica J Alvarez-Busto1, K Garcı´a-Etxebarria1, J Herrero2,3, I Garin4 and BM Jugo1 1Genetika, Antropologia Fisikoa eta Animali Fisiologia Saila, Zientzia eta Teknologia Fakultatea, Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain; 2Departamento de Ecologı´a, Universidad de Alcala´ de Henares, Alcala´ de Henares, Spain; 3EGA Wildlife Consultants, Zaragoza, Spain and 4Zoologı´a eta Animali Zelulen Biologia Saila, Zientzia eta Teknologia Fakultatea, Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain Major histocompatibility complex class II locus DRB variation recent parasitic infections by sarcoptic mange. A phyloge- was investigated by single-strand conformation polymorph- netic analysis of both Pyrenean chamois and DRB alleles ism analysis and sequence analysis in the two subspecies of from 10 different caprinid species revealed that the chamois Pyrenean chamois (Rupicapra pyrenaica) endemic to the alleles form two monophyletic groups. In comparison with Iberian Peninsula. Low levels of genetic variation were other Caprinae DRB sequences, the Rupicapra alleles detected in both subspecies, with seven different alleles in R. displayed a species-specific clustering that reflects a large p. pyrenaica and only three in the R. p. parva. After applying temporal divergence of the chamois from other caprinids, as the rarefaction method to cope with the differences in sample well as a possible difference in the selective environment for size, the low allele number of parva was highlighted. -

Cognitive Inferences in Fossil Apes (Primates, Hominoidea): Does Encephalization Reflect Intelligence?
JASs Invited Reviews Journal of Anthropological Sciences Vol. 88 (2010), pp. 11-48 Cognitive inferences in fossil apes (Primates, Hominoidea): does encephalization reflect intelligence? David M. Alba Institut Català de Paleontologia, Universitat Autònoma de Barcelona. Edifici ICP, Campus de la UAB s/n, 08193 Cerdanyola del Vallès, Barcelona (Spain); Dipartimento di Scienze della Terra, Università degli Studi di Firenze. Via G. La Pira 4, 50121 Firenze (Italy); e-mail: [email protected] Summary – Paleobiological inferences on general cognitive abilities (intelligence) in fossil hominoids strongly rely on relative brain size or encephalization, computed by means of allometric residuals, quotients or constants. This has been criticized on the basis that it presumably fails to reflect the higher intelligence of great apes, and absolute brain size has been favored instead. Many problems of encephalization metrics stem from the decrease of allometric slopes towards lower taxonomic level, thus making it difficult to determine at what level encephalization metrics have biological meaning. Here, the hypothesis that encephalization can be used as a good neuroanatomical proxy for intelligence is tested at two different taxonomic levels. A significant correlation is found between intelligence and encephalization only at a lower taxonomic level, i.e. on the basis of a low allometric slope, irrespective of whether species data or independent contrasts are employed. This indicates that higher-level slopes, resulting from encephalization grade shifts between subgroups (including hylobatids vs. great apes), do not reflect functional equivalence, whereas lower-level metrics can be employed as a paleobiological proxy for intelligence. Thus, in accordance to intelligence rankings, lower-level metrics indicate that great apes are more encephalized than both monkeys and hylobatids. -

Nesiotites Sample
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio Universidad de Zaragoza Molecular phylogenetics supports the origin of an endemic Balearic shrew lineage (Nesiotites) coincident with the Messinian Salinity Crisis Pere Bovera,b,c*, Kieren J. Mitchella, Bastien Llamasa, Juan Rofesd, Vicki A. Thomsone, Gloria Cuenca-Bescósf, Josep A. Alcoverb,c, Alan Coopera, Joan Ponsb a Australian Centre for Ancient DNA (ACAD), School of Biological Sciences, University of Adelaide, Australia b Departament de Biodiversitat i Conservació, Institut Mediterrani d’Estudis Avançats (CSIC-UIB), Esporles, Illes Balears, Spain c Research Associate, Department of Mammalogy/Division of Vertebrate Zoology, American Museum of Natural History, NY d Archéozoologie, Archéobotanique: Sociétés, pratiques et environnements (UMR 7209), Sorbonne Universités, Muséum national d'Histoire naturelle, CNRS, CP56, 55 rue Buffon, 75005 Paris, France. e School of Biological Sciences, University of Adelaide, Australia. f Grupo Aragosaurus-IUCA, Universidad de Zaragoza, Spain. * Corresponding author at: Australian Centre for Ancient DNA (ACAD), School of Biological Sciences, University of Adelaide, Darling Building, North Terrace Campus, Adelaide, SA, 5005, Australia (P. Bover). E-mail addresses: [email protected] (P. Bover), [email protected] (K.J. Mitchell), [email protected] (B. Llamas), [email protected] (J. Rofes), [email protected] (V. Thomson), [email protected] (G. Cuenca-Bescós), [email protected] (J.A. Alcover), [email protected] (A. Cooper), [email protected] (J. Pons). Abstract The red-toothed shrews (Soricinae) are the most widespread subfamily of shrews, distributed from northern South America to North America and Eurasia. -

Knowledge of the Evolution of African Paleogene Mammals
Knowledge of the évolution of African Paleogene mammals Contribution of the Bir El Ater locality (Eocène, Algeria) Rodolphe Tabuce Brigitte Coiffait Philippe-Emmanuel Coiffait Mohamed Mahboubi Jean-Jacques Jaeger 1 1 Introduction: The Early African Paleogene mammals The African fossil record of therians begins with the Early Cretaceous ofthe Middle Atlas (Morocco) (Sigogneau-Russell, 1991); however, the modem mammalian orders appear only during the early Tertiary in North Africa (figure 1A). The Paleocene and Ypresian localities from the Ouarzazate Basin (Morocco) hâve yielded mammalian faunas with possible creodonts, « insectivores » (paleoryctids, todralestids and adapisoriculids) (Gheerbrant, 1992, 1994, 1995), the oldest représentative ofeupri- mates (Sigé et al, 1990) and archaic ungulates (Sudre et al, 1993). Recently, the discovery of Phosphatherium (Proboscidea) in J\) .... Ypresian Early to Mlddla Mlddlalo Lata C> mlcldla BOCena eocena Ialaeocene eocena "III N'Tagourt2 ElKohol GllbZagdou GourLazib Chambl lnTaliclel M'Bodlone Bir el Ater DoralTalha Qaar (Moroc:co) (Algerla) (Algerla) (Algerla) (Tunlala) and (Sanagal) (Algarla) ·Evaporlt e1Sagha lamagullell Unit· (Fayum, (Mali) (LIbye) Egypt) Matatharla Kassarinolherium tunisiansa Creodonta Koholis gen. el sp. Aprarodon stlasansa indet Hyaanodon Carnlvora Glibzagdouis I8balbslsensis Condylarthra Condyfarthra Condylarthra Inda!. Inda!. Artlodaetyla cl.Bolhrio- ganys sp. Proboscldell KhSmsBcornJS Numidotherium Moerilharium Moenlherium Moarilherium 8srytherium 8srythenum buJbosus -

Evidence for an Asian Origin of Stem Anthropoids
Evidence for an Asian origin of stem anthropoids Richard F. Kay1 Department of Evolutionary Anthropology, Duke University, Durham, NC 27708-03083 n PNAS, Chaimanee et al. (1) report Genetic, embryological, and anatomical We are not there yet. Recent comprehen- a previously undescribed species of evidence demonstrates that the sister sive phylogenetic analyses stemming from I primate, Afrasia, from the late Middle group of Anthropoidea is the south Asian virtually the same datasets yield somewhat Eocene of Burma. They identify tarsier, with the two forming the crown different cladograms that reflect sensitivity Afrasia as the sister taxon to the African group Haplorhini (6–8). The other clade of to which taxa are included in the analysis genus Afrotarsius but slightly more primitive extant primates is the lemurs and lorises, and which sets of analytical assumptions are than it and allied with stem Anthropoidea called Strepsirrhini. Eocene Holarctic selected. Pertinent to the biogeographic of south Asia. Anthropoidea is the taxo- Omomyoidea are generally considered as conclusions of Chaimanee et al. (1), Seiffert nomic group that today includes New and stem haplorhines, although the precise et al. (11) conclude that Afrotarsius cha- Old World monkeys, apes, and humans. If relationship of omomyoids to tarsiers and trathi [a younger species than the one that upheld, the biogeographic significance of anthropoids is uncertain (8). Tarsius often Chaimanee et al. (1) describe] is an African these results is profound: If Afrasia and is considered to be a relictual omomyoid tarsioid. If Seiffert et al. (11) are correct, Afrotarsius are as closely related as Chai- in south Asia. -

First Fossil Evidence for the Advance of Replacement Teeth Coupled with Life History Evolution Along an Anagenetic Mammalian Lineage
First Fossil Evidence for the Advance of Replacement Teeth Coupled with Life History Evolution along an Anagenetic Mammalian Lineage Xavier Jordana1*, Nekane Marı´n-Moratalla1, Blanca Moncunill-Sole´ 1, Pere Bover2,3, Josep Antoni Alcover2,3, Meike Ko¨ hler4,5 1 Institut Catala` de Paleontologia Miquel Crusafont (ICP), Universitat Auto`noma de Barcelona, Barcelona, Catalunya, Spain, 2 Departament de Biodiversitat i Conservacio´, Institut Mediterrani d’studis Avanc¸ats (CSIC-UIB), Esporles, Illes Balears, Spain, 3 Research Associate, Division of Vertebrate Zoology/Mammalogy Department, American Museum of Natural History, New York, New York, United States of America, 4 ICREA (Catalan Institution for Research and Advanced Studies) at Institut Catala` de Paleontologia Miquel Crusafont (ICP), Universitat Auto`noma de Barcelona, Barcelona, Catalunya, Spain, 5 Department of Ecology, Universitat de Barcelona, Barcelona, Catalunya, Spain Abstract In mammals that grow up more slowly and live longer, replacement teeth tend to appear earlier in sequence than in fast growing mammals. This trend, known as ‘Schultz’s Rule’, is a useful tool for inferring life histories of fossil taxa. Deviations from this rule, however, suggest that in addition to the pace of life history, ecological factors may also drive dental ontogeny. Myotragus balearicus is an extinct insular caprine that has been proved to be an excellent test case to correlate morphological traits with life history. Here we show that Myotragus balearicus exhibits a slow signature of dental eruption sequence that is in agreement with the exceptionally slow life history of this species, thus conforming to ‘Schultz’s Rule’. However, our results also show an acceleration of the absolute pace of development of the permanent incisors in relation to that of the posterior teeth. -

Evolutionary History of Lorisiform Primates
Evolution: Reviewed Article Folia Primatol 1998;69(suppl 1):250–285 oooooooooooooooooooooooooooooooo Evolutionary History of Lorisiform Primates D. Tab Rasmussen, Kimberley A. Nekaris Department of Anthropology, Washington University, St. Louis, Mo., USA Key Words Lorisidae · Strepsirhini · Plesiopithecus · Mioeuoticus · Progalago · Galago · Vertebrate paleontology · Phylogeny · Primate adaptation Abstract We integrate information from the fossil record, morphology, behavior and mo- lecular studies to provide a current overview of lorisoid evolution. Several Eocene prosimians of the northern continents, including both omomyids and adapoids, have been suggested as possible lorisoid ancestors, but these cannot be substantiated as true strepsirhines. A small-bodied primate, Anchomomys, of the middle Eocene of Europe may be the best candidate among putative adapoids for status as a true strepsirhine. Recent finds of Eocene primates in Africa have revealed new prosimian taxa that are also viable contenders for strepsirhine status. Plesiopithecus teras is a Nycticebus- sized, nocturnal prosimian from the late Eocene, Fayum, Egypt, that shares cranial specializations with lorisoids, but it also retains primitive features (e.g. four premo- lars) and has unique specializations of the anterior teeth excluding it from direct lorisi- form ancestry. Another unnamed Fayum primate resembles modern cheirogaleids in dental structure and body size. Two genera from Oman, Omanodon and Shizarodon, also reveal a mix of similarities to both cheirogaleids and anchomomyin adapoids. Resolving the phylogenetic position of these Africa primates of the early Tertiary will surely require more and better fossils. By the early to middle Miocene, lorisoids were well established in East Africa, and the debate about whether these represent lorisines or galagines is reviewed. -
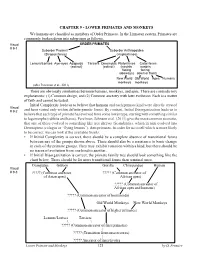
CHAPTER 9 - LOWER PRIMATES and MONKEYS We Humans Are Classified As Members of Order Primates
CHAPTER 9 - LOWER PRIMATES AND MONKEYS We humans are classified as members of Order Primates. In the Linnaean system, Primates are commonly broken down into subgroups as follows. Visual ORDER PRIMATES # 9-1 Suborder Prosimii Suborder Anthropoidea (Strepsirrhines) (Haplorrhines) Lemurs/Lorises Aye-ayes Adapoids Tarsiers Omomyids Platyrrhines Catarrhines (extinct) (extinct) (nostrils nostrils facing facing sideways) down or front) New World Old World Apes Humans monkeys monkeys (after Perelman et al., 2011) There are obviously similarities between humans, monkeys, and apes. There are contradictory explanations: (1) Common design, and (2) Common ancestry with later evolution. Each is a matter of faith and cannot be tested. Initial Complexity leads us to believe that humans and each primate kind were directly created Visual # 9-2 and have varied only within definite genetic limits. By contrast, Initial Disorganization leads us to believe that each type of primate has evolved from some lower type, starting with something similar to lagomorphs (rabbits and hares). Perelman, Johnson et al. (2011) give the most common scenario, that one of these evolved to something like tree shrews (Scandentia), which in turn evolved into Dermoptera (colugos or “flying lemurs”), then primates. In order for us to tell which is more likely to be correct, we can look at the available fossils. • If Initial Complexity is correct, there should be a complete absence of transitional forms between any of the groups shown above. There should also be a resistance to basic change in each of the primate groups. They may exhibit variation within a kind, but there should be no traces of evolution from one kind to another.