(12) Patent Application Publication (10) Pub. No.: US 2003/0056896 A1 Jao Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Lab Standard Operating Procedures (Sops)
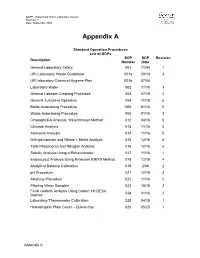
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

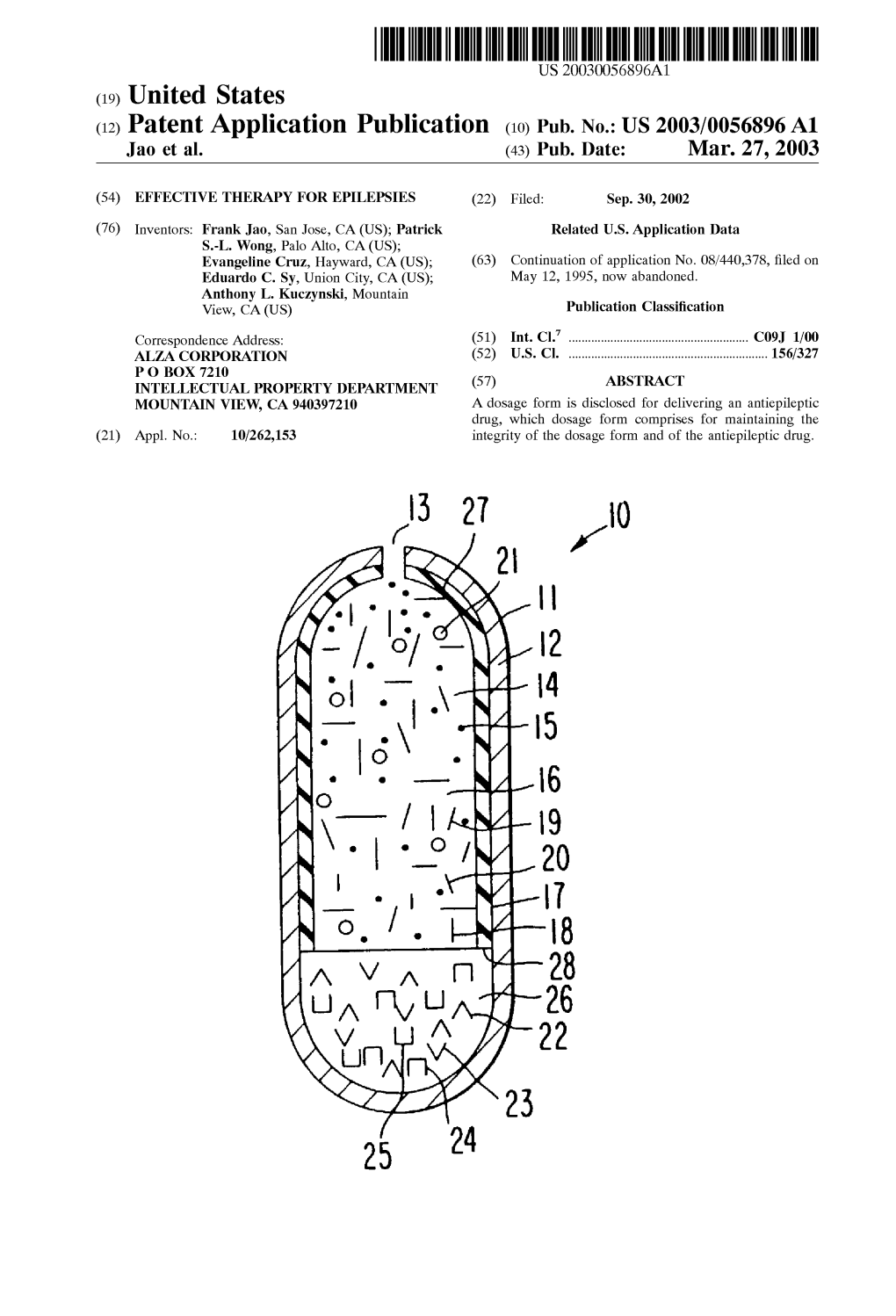
(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on Β-Alanine and Isatin
Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin by Robert Philip Colaguori A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Pharmaceutical Sciences University of Toronto © Copyright by Robert Philip Colaguori, 2016 ii Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin Robert Philip Colaguori Master of Science Department of Pharmaceutical Sciences University of Toronto 2016 Abstract Epilepsy is the fourth-most common neurological disorder in the world. Approximately 70% of cases can be controlled with therapeutics, however 30% remain pharmacoresistant. There is no cure for the disorder, and patients affected are subsequently medicated for life. Thus, there is a need to develop compounds that can treat not only the symptoms, but also delay/prevent progression. Previous work resulted in the discovery of NC-2505, a substituted β-alanine with activity against chemically induced seizures. Several N- and α-substituted derivatives of this compound were synthesized and evaluated in the kindling model and 4-AP model of epilepsy. In the kindling model, RC1-080 and RC1-102 were able to decrease the mean seizure score from 5 to 3 in aged mice. RC1-085 decreased the interevent interval by a factor of 2 in the 4-AP model. Future studies are focused on the synthesis of further compounds to gain insight on structure necessary for activity. iii Acknowledgments First and foremost, I would like to thank my supervisor Dr. Donald Weaver for allowing me to join the lab as a graduate student and perform the work ultimately resulting in this thesis. -

Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms
International Journal of Molecular Sciences Article Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)—A New Anticonvulsant Drug Candidate—On Living Organisms Anna Makuch-Kocka 1,* , Marta Andres-Mach 2, Mirosław Zagaja 2, Anna Smiech´ 3 , Magdalena Pizo ´n 4 , Jolanta Flieger 4, Judyta Cielecka-Piontek 5 and Tomasz Plech 1 1 Department of Pharmacology, Faculty of Health Sciences, Medical University of Lublin, 20-093 Lublin, Poland; [email protected] 2 Isobolographic Analysis Laboratory, Institute of Rural Health, 20-090 Lublin, Poland; [email protected] (M.A.-M.); [email protected] (M.Z.) 3 Sub-Department of Pathomorphology and Forensic Veterinary Medicine, Department and Clinic of Animal Internal Diseases, University of Life Sciences in Lublin, 20-612 Lublin, Poland; [email protected] 4 Department of Analytical Chemistry, Faculty of Pharmacy, Medical University of Lublin, 20-093 Lublin, Poland; [email protected] (M.P.); jolanta.fl[email protected] (J.F.) 5 Department of Pharmacognosy, Faculty of Pharmacy, Poznan University of Medical Sciences, 61-781 Pozna´n,Poland; [email protected] * Correspondence: [email protected] Citation: Makuch-Kocka, A.; Andres-Mach, M.; Zagaja, M.; Smiech,´ Abstract: About 70 million people suffer from epilepsy—a chronic neurodegenerative disease. In A.; Pizo´n,M.; Flieger, J.; most cases, the cause of the disease is unknown, but epilepsy can also develop as the result of a Cielecka-Piontek, J.; Plech, T. Effect of Chronic Administration of stroke, trauma to the brain, or the use of psychotropic substances. -

Pharmaceutical Composition Comprising Brivaracetam and Lacosamide with Synergistic Anticonvulsant Effect
(19) TZZ __T (11) EP 2 992 891 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 09.03.2016 Bulletin 2016/10 A61K 38/04 (2006.01) A61K 31/4015 (2006.01) A61P 25/08 (2006.01) (21) Application number: 15156237.8 (22) Date of filing: 15.06.2007 (84) Designated Contracting States: (71) Applicant: UCB Pharma GmbH AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 40789 Monheim (DE) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR (72) Inventor: STOEHR, Thomas 2400 Mol (BE) (30) Priority: 15.06.2006 US 813967 P 12.10.2006 EP 06021470 (74) Representative: Dressen, Frank 12.10.2006 EP 06021469 UCB Pharma GmbH 22.11.2006 EP 06024241 Alfred-Nobel-Strasse 10 40789 Monheim (DE) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: Remarks: 07764676.8 / 2 037 965 This application was filed on 24-02-2015 as a divisional application to the application mentioned under INID code 62. (54) PHARMACEUTICALCOMPOSITION COMPRISING BRIVARACETAM AND LACOSAMIDE WITH SYNERGISTIC ANTICONVULSANT EFFECT (57) The present invention is directed to a pharmaceutical composition comprising (a) lacosamide and (b) brivara- cetam for the prevention, alleviation or/and treatment of epileptic seizures. EP 2 992 891 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 992 891 A1 Description [0001] The present application claims the priorities of US 60/813.967 of 15 June 2006, EP 06 021 470.7 of 12 October 2006, EP 06 021 469.9 of 12 October 2006, and EP 06 024 241.9 of 22 November 2006, which are included herein by 5 reference. -

Diazepam Therapy and CYP2C19 Genotype
NLM Citation: Dean L. Diazepam Therapy and CYP2C19 Genotype. 2016 Aug 25. In: Pratt VM, McLeod HL, Rubinstein WS, et al., editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Bookshelf URL: https://www.ncbi.nlm.nih.gov/books/ Diazepam Therapy and CYP2C19 Genotype Laura Dean, MD1 Created: August 25, 2016. Introduction Diazepam is a benzodiazepine with several clinical uses, including the management of anxiety, insomnia, muscle spasms, seizures, and alcohol withdrawal. The clinical response to benzodiazepines, such as diazepam, varies widely between individuals (1, 2). Diazepam is primarily metabolized by CY2C19 and CYP3A4 to the major active metabolite, desmethyldiazepam. Approximately 3% of Caucasians and 15 to 20% of Asians have reduced or absent CYP2C19 enzyme activity (“poor metabolizers”). In these individuals, standard doses of diazepam may lead to a higher exposure to diazepam. The FDA-approved drug label for diazepam states that “The marked inter-individual variability in the clearance of diazepam reported in the literature is probably attributable to variability of CYP2C19 (which is known to exhibit genetic polymorphism; about 3-5% of Caucasians have little or no activity and are “poor metabolizers”) and CYP3A4” (1). Drug: Diazepam Diazepam is used in the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. In acute alcohol withdrawal, diazepam may provide symptomatic relief from agitation, tremor, delirium tremens, and hallucinations. Diazepam is also useful as an adjunct treatment for the relief of acute skeletal muscle spasms, as well as spasticity caused by upper motor neuron disorders (3). There are currently 16 benzodiazepines licensed by the FDA. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

General Pharmacology
GENERAL PHARMACOLOGY Winners of “Nobel” prize for their contribution to pharmacology Year Name Contribution 1923 Frederick Banting Discovery of insulin John McLeod 1939 Gerhard Domagk Discovery of antibacterial effects of prontosil 1945 Sir Alexander Fleming Discovery of penicillin & its purification Ernst Boris Chain Sir Howard Walter Florey 1952 Selman Abraham Waksman Discovery of streptomycin 1982 Sir John R.Vane Discovery of prostaglandins 1999 Alfred G.Gilman Discovery of G proteins & their role in signal transduction in cells Martin Rodbell 1999 Arvid Carlson Discovery that dopamine is neurotransmitter in the brain whose depletion leads to symptoms of Parkinson’s disease Drug nomenclature: i. Chemical name ii. Non-proprietary name iii. Proprietary (Brand) name Source of drugs: Natural – plant /animal derivatives Synthetic/semisynthetic Plant Part Drug obtained Pilocarpus microphyllus Leaflets Pilocarpine Atropa belladonna Atropine Datura stramonium Physostigma venenosum dried, ripe seed Physostigmine Ephedra vulgaris Ephedrine Digitalis lanata Digoxin Strychnos toxifera Curare group of drugs Chondrodendron tomentosum Cannabis indica (Marijuana) Various parts are used ∆9Tetrahydrocannabinol (THC) Bhang - the dried leaves Ganja - the dried female inflorescence Charas- is the dried resinous extract from the flowering tops & leaves Papaver somniferum, P album Poppy seed pod/ Capsule Natural opiates such as morphine, codeine, thebaine Cinchona bark Quinine Vinca rosea periwinkle plant Vinca alkaloids Podophyllum peltatum the mayapple -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine