Kinetics and Muscle Modeling of a Single Degree of Freedom Joint Part II: Spindles and Sensory Neurons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Somatosensory Systems
Somatosensory Systems Sue Keirstead, Ph.D. Assistant Professor Dept. of Integrative Biology and Physiology Stem Cell Institute E-mail: [email protected] Tel: 612 626 2290 Class 9: Somatosensory System (p. 292-306) 1. Describe the 3 main types of somatic sensations: 1. tactile: light touch, deep pressure, vibration, cold, hot, etc., 2. pain, 3. Proprioception. 2. List the types of sensory receptors that are found in the skin (Figure 9.11) and explain what determines the optimum type of stimulus that will activate each. 3. Describe the two different modality-specific ascending somatosensory pathways and note which modalities are carried in each (Figure 9.10 and 9.13). 4. Describe how it is possible for us to differentiate between stimuli of different modalities in the same body part (i.e. fingertip). Consider this at the level of 1) the sensory receptors and 2) the neurons onto which they synapse in the ascending sensory systems. 5. Explain how one might determine the location of a spinal cord injury based on the modality of sensation that is lost and the region of the body (both the side of the body and body part) where sensation is lost (Figure 9.18). 6. Describe how incoming sensory inputs from primary sensory axons can be modified at the level of the spinal cord and relate this to the mechanism of action of some common pain medications (Figure 9-18). 7. Describe the homunculus and explain the significance of the size of the region of the somatosensory cortex devoted to a particular body part. Cerebral cortex Interneuron Thalamus Interneuron 4 Integration of sensory Stimulus input in the CNS 1 Stimulation Sensory Axon of sensory of sensory receptor neuron receptor Graded potential Action potentials 2 Transduction 3 Generation of of the stimulus action potentials Copyright © 2016 by John Wiley & Sons, Inc. -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

BRS Physiology 3Rd Edition
Board Review Series • Reflects USMLE changes • Approximately 350 USMLE-type questions with explanations • Numerous illustrations, diagrams, and tables • Easy-to-follow outline covering all USMLE-tested topics • A comprehensive examination V Ah, LIPPINCOTT -"*" WILLIAMS &WILKIN; mum IEEE mows IF 'IMP IMMO MINK I I. Key Physiology Topics for USMLE Step I Cell Physiology Transport mechanisms Ionic basis for action potential Excitation-contraction coupling in skeletal, cardiac, and smooth muscle Neuromuscular transmission Autonomic Physiology Cholinergic receptors Adrenergic receptors Effects of autonomic nervous system on organ system function Cardiovascular Physiology Events of cardiac cycle Pressure, flow, resistance relationships Frank-Starling law of the heart Ventricular pressure-volume loops Ionic basis for cardiac action potentials Starling forces in capillaries Regulation of arterial pressure (baroreceptors and renin-angiotensin II-aldosterone system) Cardiovascular and pulmonary responses to exercise Cardiovascular responses to hemorrhage Cardiovascular responses to changes in posture Respiratory Physiology Lung and chest-wall compliance curves Breathing cycle Hemoglobin-02 dissociation curve Causes of hypoxemia and hypoxia vq, P02, and P00 2 in upright lung V/Q defects Peripheral and central chemoreceptors in control of breathing Responses to high altitude Renal and Acid-Base Physiology Fluid shifts between body fluid compartments Starling forces across glomerular capillaries Transporters in various segments of nephron (Na Cl-, -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

The Human Nervous System S Tructure and Function
The Human Nervous System S tructure and Function S ixth Ed ition The Human Nervous System Structure and Function S ixth Edition Charles R. Noback, PhD Professor Emeritus Department of Anatomy and Cell Biology College of Physicians and Surgeons Columbia University, New York, NY Norman L. Strominger, PhD Professor Center for Neuropharmacology and Neuroscience Department of Surgery (Otolaryngology) The Albany Medical College Adjunct Professor, Division of Biomedical Science University at Albany Institute for Health and the Environment Albany, NY Robert J. Demarest Director Emeritus Department of Medical Illustration College of Physicians and Surgeons Columbia University, New York, NY David A. Ruggiero, MA, MPhil, PhD Professor Departments of Psychiatry and Anatomy and Cell Biology Columbia University College of Physicians and Surgeons New York, NY © 2005 Humana Press Inc. 999 Riverview Drive, Suite 208 Totowa, New Jersey 07512 www.humanapress.com All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording, or otherwise without written permission from the Publisher. All papers, comments, opinions, conclusions, or recommendations are those of the author(s), and do not necessarily reflect the views of the publisher. This publication is printed on acid-free paper. h ANSI Z39.48-1984 (American Standards Institute) Permanence of Paper for Printed Library Materials. Production Editor: Tracy Catanese Cover design by Patricia F. Cleary Cover Illustration: The cover illustration, by Robert J. Demarest, highlights synapses, synaptic activity, and synaptic-derived proteins, which are critical elements in enabling the nervous system to perform its role. -

Malak Shalfawi Noor Adnan Lina Abdelhadi Fasial Mohammad
9 Noor Adnan Malak Shalfawi Lina abdelhadi Fasial Mohammad 0 Motor system – Motor of the spinal cord Before we start talking about the motor function of the spinal cord, let’s first take a quick look at the motor system and the incredible connections taking place inside it: [please refer to the pictures for better understanding] 1- Motor command For any motor function (movement) to occur, the nervous system has motor command that comes from the cerebral motor cortex to the spinal cord and these descending tracts (corticospinal) are called also pyramidal tracts and that’s because they pass through the pyramids of medulla oblongata. The neuronal fibers coming from the cortex ending in the spinal cord are considered upper motor neurons, while the neuronal fibers going out from the spinal cord to reach the muscles are called lower motor neurons. There are other origins for the motor commands such as the brain stem and the red nucleus that send neuronal fibers to the spinal cord in order to control the activity of the muscles. 2- Motor command intension At the same time, there are some tracts going from the cortex to the cerebellum through the brain stem like the corticopontocerebellar, corticoreticulocerebellar, corticolivarycerebellar tracts. And these tracts are telling the cerebellum about the intended movements. (= the movements we want to do) 3-motor command monitor/ feedback system a- Inside the muscles we have receptors (muscle spindles/ stretch receptors, Golgi tendon organs) that are connected to sensory (afferent) neuronal fibers that goes to the spinal cord relay nuclei. 1 | P a g e b- From the spinal cord they go to the cerebellum through ventral and dorsal spinocerebellar tracts to tell the cerebellum what is exactly happening down at the level of the muscles. -
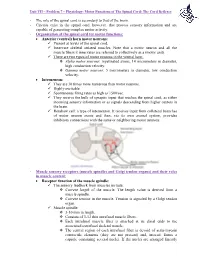
The Role of the Spinal Cord Is Secondary to That of the Brain
Unit VII – Problem 7 – Physiology: Motor Functions of The Spinal Cord; The Cord Reflexes - The role of the spinal cord is secondary to that of the brain. - Circuits exist in the spinal cord, however, that process sensory information and are capable of generating complex motor activity. - Organization of the spinal cord for motor functions: Anterior (ventral) horn motor neurons: Present at levels of the spinal cord. Innervate skeletal striated muscles. Note that a motor neuron and all the muscle fibers it innervates are referred to collectively as a (motor unit). There are two types of motor neurons in the ventral horn: Alpha motor neurons: myelinated axons, 14 micrometers in diameter, high conduction velocity. Gamma motor neurons: 5 micrometers in diameter, low conduction velocity. Interneuron: They are 30 times more numerous than motor neurons. Highly excitable. Spontaneous firing rates as high as 1500/sec. They receive the bulk of synaptic input that reaches the spinal cord, as either incoming sensory information or as signals descending from higher centers in the brain. Renshaw cell: a type of interneuron. It receives input from collateral branches of motor neuron axons and then, via its own axonal system, provides inhibitory connections with the same or neighboring motor neurons. - Muscle sensory receptors (muscle spindles and Golgi tendon organs) and their roles in muscle control: Receptor function of the muscle spindle: The sensory feedback from muscles include: Current length of the muscle. The length value is derived from a muscle spindle. Current tension in the muscle. Tension is signaled by a Golgi tendon organ. Muscle spindle: 3-10 mm in length. -

Motor System 1: Spinal Cord
10353-11_CH11.qxd 8/30/07 1:16 PM Page 238 11 Motor System 1: Spinal Cord LEARNING OBJECTIVES This cortical control provides a type of parallel process- ing with rostral domination of hierarchical motor organiza- After studying this chapter, students should be able to: tion and simultaneous control of the segmental output itself. Because some time is involved in the conduction of afferent • Discuss the anatomy of the spinal cord information, local (spinal) reflexes often are activated first, • Describe the functions of the spinal structures and motor mechanisms at higher hierarchic levels are acti- vated slightly later. The best example of reflexive move- • Discuss the importance of the motor unit in movement ment is mistakenly stepping on a tack with your bare foot. • List the major ascending and descending spinal tracts This triggers a leg-withdrawal reflex, which is already in progress before the forebrain can inhibit or modify the • Describe the functions of the major sensorimotor tracts action. However, the higher motor systems can inhibit • Explain the role of muscle spindles in reflexive motor such a withdrawal reflex if the stimulus is expected and functions another response has been learned. For example, if some- one has to pick up a hot cup, the withdrawal reflex can be • Discuss the importance of the Golgi tendon organ inhibited while the hot cup is being moved to a support- • Describe the physiology of basic spinal reflexes ing surface, even though the fingers are being burned. The hierarchically defined motor functions and the • Explain the importance of the lower motor neurons nature of the specific contributions by each level are dis- • Discuss lower motor neuron syndrome cussed in Chapters 11–14. -
(Neuron) (Spinal Cord and Brain) Neuroglia (Support) Neuroglia
210 اوم څپرکې عصبي نسج (NERVOUS TISSUE) (impulse) (neuron) (spinal cord and brain) neuroglia (support) neuroglia ٧- ١ شکل دماغ 1012 211 اوم څپرکې عصبي نسج (axons) (NEURON STRUCTURE) cell body Cell body perikaryon Soma ٧-٢ شکل دنيوران جوړښت centrioles granules basal body nissl substance 212 اوم څپرکې عصبي نسج Endoplasmic Reticulum neurotransmitters neurofibrils microtubules microfilaments neurofibrils microtubules substantia (pigmented granules) nerumelanin nigra lipofuscin cell body neuritis Dendrites Axons cell body Dendrites nissle dendrites dendrites granules dendrites cell body 213 اوم څپرکې عصبي نسج axon nissle granuls (axon and dendrites) axon cell body dendrites cell body MAP-2 dendrites dendrites nissl granules axon cell body cell body initial segments axon hillock hillock Schwann cells (sheath) oligodendrocytes Schwann cells Schwann cell ٧- ٤ شکل Schwann cells mesaxon 214 اوم څپرکې عصبي نسج (mesaxon) mesaxon myelin sheath schwann cells myelin sheath neurolimmal sheath neurilemma Schwann cell sheath myelinated axons (myelin sheath) axons Schwann cells myelin sheath myelin 215 اوم څپرکې عصبي نسج node of Ranvier myelin sheath (nodes of Ranvier) (Internode) unmyelinated axons myelin sheath mesaxon Schwann cells Schwann cell body collaterals telodendria boutons terminal boutons of terminaux synapse ganglions synapse 216 اوم څپرکې عصبي نسج dendrites 6,8,3 (nerve fibers) cell body cell bodies cell bodies 120µm cell body 5µm ٧-٨ شکل داکسون او cell body نيوران ارتبات multipolar neurons (bipolar neuron) unipolar neuron bipolar neuron (pseudounipolar -

Human Motion Control Proprioception Open and Closed Loop Control
Proprioception Open and closed loop control • Introduction Human Motion Control – Musculoskeletal system with proprioceptive feedback 2008-2009 – Role of sensors Proprioception • Nervous system – Central nervous system – Peripheral nervous system – Sensors and neurons • Information Processing – Organization & reflexes • Sensors in motor pathways – Joint capsule sensors – Muscle spindles – Golgi Tendon Organs Proprioception Proprioception Proprioception Loss of proprioception Have you ever said any of the following? Example loss of proprioception: Ian Waterman from proprius Latin for “one’s own” and - “I think I smell gas!” - No sensory feedback below the neck perception. - “Did you hear that sound?” - Not paralyzed! - “I have a headache.” - One of only 10 patients in the world. The body's awareness of position, posture, - “I just noticed my elbow is slightly flexed and movement and changes in equilibrium my hand is approximately 30 centimeters away from my face.” The man who lost Proprioception happens unconsciously. his body 1 Sensors Central Nervous System (CNS) Structure of the CNS • 1011 neurons • Vestibulary system: Translational and rotational accelerations of the head • 104 synapses per neuron: • Visual system: Position and velocity information 1015 synapses • Tactile system: External force information • A-synchronous processing • Joint capsule receptors: Joint angle • Distributed information storage • Muscle spindles: Muscle length and contractile velocity • Golgi tendon organs: Muscle Force Sensory integration Central Nervous System CNS Sensory and motor projections Sensory and motor projections The Brain in the cortex in the cortex Cerebrum Higher brain functions like: speech, emotions, reasoning, movements, vision, memory, etc… Cerebellum Brain stem Regulation and coordination Basic vital life functions of movement, posture and like breathing, heartbeat, balance. -

Muscle Receptors, Spinal Reflexes and Muscles
Muscle Receptors, Spinal Reflexes and Muscles 0 http://www.tutis.ca/NeuroMD/index.htm 19 February 2013 Contents Muscle Receptors, Spinal Reflexes and Muscles ............................................................... 0 Contents .......................................................................................................................... 1 Introduction ..................................................................................................................... 2 Why are the location of muscle spindles and Golgi tendon organs different? ............... 3 Compare the response of spindles and golgi tendon organs. .......................................... 4 Gamma drive controls the sensitivity of the muscle spindle. ......................................... 5 Three spinal reflexes ....................................................................................................... 6 1. The monosynaptic stretch reflex is activated by Ia spindle afferents. ....................... 6 2. The reflex mediated by the Golgi tendon organ (Ib) .................................................. 7 3. Withdraw reflexes mediated by pain receptors ........................................................... 7 The pathway for transmission of proprioception to the primary somatosensory cortex . 8 A muscle consists of many motor units. .......................................................................... 9 The fiber types and their distinguishing features .......................................................... 10 How is the -
Atlas.Of.Neuroanatomy.And.Neurophysiology.Frank.H.Netter
Atlas of Neuroanatomy and Neurophysiology Selections from the Netter Collection of Medical Illustrations Illustrations by Frank H. Netter, MD John A. Craig, MD James Perkins, MS, MFA Text by John T. Hansen, PhD Bruce M. Koeppen, MD, PhD Atlas of Neuroanatomy and Neurophysiology Selections from the Netter Collection of Medical Illustrations Copyright ©2002 Icon Custom Communications. All rights reserved. The contents of this book may not be reproduced in any form without written authorization from Icon Custom Communications. Requests for permission should be addressed to Permissions Department, Icon Custom Communications, 295 North St., Teterboro NJ 07608, or can be made at www. Netterart.com. NOTICE Every effort has been taken to confirm the accuracy of the information presented. Neither the publisher nor the authors can be held responsible for errors or for any consequences arising from the use of the information contained herein, and make no warranty, expressed or implied, with respect to the contents of the publication. Printed in U.S.A. Foreword Frank Netter: The Physician, The Artist, The Art This selection of the art of Dr. Frank H. Netter on neuroanatomy and neurophysiology is drawn from the Atlas of Human Anatomy and Netter’s Atlas of Human Physiology. Viewing these pictures again prompts reflection on Dr. Netter’s work and his roles as physician and artist. Frank H. Netter was born in 1906 in New York City. He pursued his artistic muse at the Sorbonne, the Art Student’s League, and the National Academy of Design before entering medical school at New York University, where he received his M.D.