Phosphatidylinositol 4,5-Bisphosphate Modifies Tubulin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mito
fnins-12-00402 June 26, 2018 Time: 12:46 # 1 ORIGINAL RESEARCH published: 26 June 2018 doi: 10.3389/fnins.2018.00402 Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mitochondrial Trafficking in Adult Sensory Neurons Mohammad G. Sabbir1*, Nigel A. Calcutt2 and Paul Fernyhough1,3 1 Division of Neurodegenerative Disorders, St. Boniface Hospital Research Centre, Winnipeg, MB, Canada, 2 Department of Pathology, University of California, San Diego, San Diego, CA, United States, 3 Department of Pharmacology and Therapeutics, University of Manitoba, Winnipeg, MB, Canada The muscarinic acetylcholine type 1 receptor (M1R) is a metabotropic G protein-coupled Edited by: receptor. Knockout of M1R or exposure to selective or specific receptor antagonists Roberto Di Maio, elevates neurite outgrowth in adult sensory neurons and is therapeutic in diverse University of Pittsburgh, United States models of peripheral neuropathy. We tested the hypothesis that endogenous M1R Reviewed by: activation constrained neurite outgrowth via a negative impact on the cytoskeleton Roland Brandt, University of Osnabrück, Germany and subsequent mitochondrial trafficking. We overexpressed M1R in primary cultures Rick Dobrowsky, of adult rat sensory neurons and cell lines and studied the physiological and The University of Kansas, United States molecular consequences related to regulation of cytoskeletal/mitochondrial dynamics *Correspondence: and neurite outgrowth. In adult primary neurons, overexpression of M1R caused Mohammad G. Sabbir disruption of the tubulin, but not actin, cytoskeleton and significantly reduced neurite [email protected] outgrowth. Over-expression of a M1R-DREADD mutant comparatively increased neurite Specialty section: outgrowth suggesting that acetylcholine released from cultured neurons interacts This article was submitted to with M1R to suppress neurite outgrowth. -
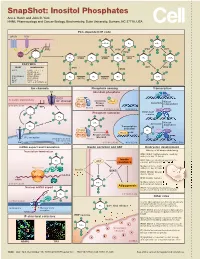
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Evidence for Conformational Change-Induced Hydrolysis of Β-Tubulin-GTP
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Evidence for conformational change-induced hydrolysis of β-tubulin-GTP Mohammadjavad Paydar1 and Benjamin H. Kwok1† 1 Institute for Research in Immunology and Cancer (IRIC), Département de médecine, Université de Montréal, P.O. Box 6128, Station Centre-Ville, Montréal, QC H3C 3J7, Canada. † Corresponding author: B. H. Kwok: [email protected] Keywords: Kinesin, Kif2A, MCAK, motor protein, microtubules, tubulin, GTP hydrolysis Abbreviations NM: Neck+Motor MD: Motor domain MT: Microtubule Running title: Tubulin GTP hydrolysis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Microtubules, protein polymers of α/β-tubulin dimers, form the structural framework for many essential cellular processes including cell shape formation, intracellular transport, and segregation of chromosomes during cell division. It is known that tubulin-GTP hydrolysis is closely associated with microtubule polymerization dynamics. However, the precise roles of GTP hydrolysis in tubulin polymerization and microtubule depolymerization, and how it is initiated are still not clearly defined. We report here that tubulin-GTP hydrolysis can be triggered by conformational change induced by the depolymerizing kinesin-13 proteins or by the stabilizing chemical agent paclitaxel. We provide biochemical evidence that conformational change precedes tubulin-GTP hydrolysis, confirming this process is mechanically driven and structurally directional. -

NIH Public Access Author Manuscript Future Neurol
NIH Public Access Author Manuscript Future Neurol. Author manuscript; available in PMC 2013 January 08. Published in final edited form as: Future Neurol. 2012 November 1; 7(6): 749–771. doi:10.2217/FNL.12.68. Opening Pandora’s jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, $watermark-textand $watermark-text central $watermark-text disorders Rajesh Khanna1,2,3,4,*, Sarah M Wilson1,‡, Joel M Brittain1,‡, Jill Weimer5, Rukhsana Sultana6, Allan Butterfield6, and Kenneth Hensley7 1Program in Medical Neurosciences, Paul & Carole Stark Neurosciences Research Institute Indianapolis, IN 46202, USA 2Departments of Pharmacology & Toxicology, Indianapolis, IN 46202, USA 3Biochemistry & Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46202, USA 4Sophia Therapeutics LLC, Indianapolis, IN 46202, USA 5Sanford Children’s Health Research Center, Sanford Research & Department of Pediatrics, Sanford School of Medicine of the University of South Dakota, Sioux Falls, SD 57104, USA 6Department of Chemistry, Center of Membrane Sciences, & Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40506, USA 7Department of Pathology & Department of Neurosciences, University of Toledo Medical Center, Toledo, OH 43614, USA Abstract CRMP2, also known as DPYSL2/DRP2, Unc-33, Ulip or TUC2, is a cytosolic phosphoprotein that mediates axon/dendrite specification and axonal growth. Mapping the CRMP2 interactome has revealed previously unappreciated functions subserved by this -

1/05661 1 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . ... - 12 May 2011 (12.05.2011) W 2 11/05661 1 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12Q 1/00 (2006.0 1) C12Q 1/48 (2006.0 1) kind of national protection available): AE, AG, AL, AM, C12Q 1/42 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 10/054171 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 26 October 2010 (26.10.2010) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/255,068 26 October 2009 (26.10.2009) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, MYREXIS, INC. -

ITPK1 Mediates the Lipid-Independent Synthesis of Inositol Phosphates Controlled by Metabolism
ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism Yann Desfougèresa, Miranda S. C. Wilsona, Debabrata Lahaa, Gregory J. Millerb, and Adolfo Saiardia,1 aMedical Research Council Laboratory for Molecular Cell Biology, University College London, WC1E 6BT London, United Kingdom; and bDepartment of Chemistry, The Catholic University of America, Washington, DC 20064 Edited by Solomon H. Snyder, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved October 25, 2019 (received for review July 3, 2019) Inositol phosphates (IPs) comprise a network of phosphorylated The potential pathways to IP6-7-8 synthesis have a profound molecules that play multiple signaling roles in eukaryotes. IPs impact on how we interpret their signaling roles. If they are synthesis is believed to originate with IP3 generated from PIP2 by synthesized from IP3, their function has evolved in relation to phospholipase C (PLC). Here, we report that in mammalian cells lipid-dependent PLC and/or calcium signaling. This constraint is PLC-generated IPs are rapidly recycled to inositol, and uncover the relieved if IP6 synthesis occurs lipid-independently, originating enzymology behind an alternative “soluble” route to synthesis of directly from inositol, a “soluble” pathway. In yeast, which do not IPs. Inositol tetrakisphosphate 1-kinase 1 (ITPK1)—found in Asgard have IP3-regulated calcium signaling, IP6 synthesis strictly depends archaea, social amoeba, plants, and animals—phosphorylates on the PLC-generated IP3 (Figs. 1A and 2D). The higher com- I(3)P1 originating from glucose-6-phosphate, and I(1)P1 generated plexity of other eukaryotes might allow different pathways for IPs from sphingolipids, to enable synthesis of IP6. -

Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus Fumigatus
Journal of Fungi Article Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus fumigatus Jizhou Li 1, Isabelle Mouyna 1, Christine Henry 1, Frédérique Moyrand 2, Christian Malosse 3, Julia Chamot-Rooke 3, Guilhem Janbon 2, Jean-Paul Latgé 1 and Thierry Fontaine 1,* 1 Unité des Aspergillus, 25 rue du Docteur Roux, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (J.L.); [email protected] (I.M.); [email protected] (C.H.); [email protected] (J.-P.L.) 2 Unité de Biologie des ARN des Pathogènes Fongiques, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (F.M.); [email protected] (G.J.) 3 Unité de Spectrométrie de Masse pour la Biologie, Institut Pasteur, CNRS USR 2000, 28 rue du Docteur Roux, 75015 Paris, France; [email protected] (C.M.); [email protected] (J.C.-R.) * Correspondence: [email protected]; Tel.: +33-145-688-358 Received: 8 December 2017; Accepted: 19 January 2018; Published: 2 Febuary 2018 Abstract: Glycosylphosphatidylinositols (GPIs) are lipid anchors allowing the exposure of proteins at the outer layer of the plasma membrane. In fungi, a number of GPI-anchored proteins (GPI-APs) are involved in the remodeling of the cell wall polymers. GPIs follow a specific biosynthetic pathway in the endoplasmic reticulum. After the transfer of the protein onto the GPI-anchor, a lipid remodeling occurs to substitute the diacylglycerol moiety by a ceramide. In addition to GPI-APs, A. fumigatus produces a GPI-anchored polysaccharide, the galactomannan (GM), that remains unique in the fungal kingdom. -

Dephosphorylation of 2,3-Bisphosphoglycerate by MIPP Expands the Regulatory Capacity of the Rapoport–Luebering Glycolytic Shunt
Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport–Luebering glycolytic shunt Jaiesoon Cho*†, Jason S. King‡§, Xun Qian*, Adrian J. Harwood‡, and Stephen B. Shears*¶ *Laboratory of Signal Transduction, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Social Services, P.O. Box 12233, Research Triangle Park, NC 27709; and ‡Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3US, United Kingdom Edited by Helen M. Ranney, University of California at San Diego, La Jolla, CA, and approved March 6, 2008 (received for review November 21, 2007) The Rapoport–Luebering glycolytic bypass comprises evolutionar- majority of mammals. By preferentially binding to deoxyhemo- ily conserved reactions that generate and dephosphorylate 2,3- globin, 2,3-BPG facilitates oxygen release from the erythrocyte bisphosphoglycerate (2,3-BPG). For >30 years, these reactions have to the surrounding tissues (6, 7). Thus, the regulation of eryth- been considered the responsibility of a single enzyme, the 2,3-BPG rocyte 2,3-BPG levels is key to efficiently meeting tissue oxygen synthase/2-phosphatase (BPGM). Here, we show that Dictyosteli- demands while also providing an important physiological adap- um, birds, and mammals contain an additional 2,3-BPG phospha- tation to oxygen deprivation (8), including that which occurs at tase that, unlike BPGM, removes the 3-phosphate. This discovery high altitude (9) or during postoperative anemia (10). reveals that the glycolytic pathway can bypass the formation of Despite the importance of carefully regulating 2,3-BPG turn- 3-phosphoglycerate, which is a precursor for serine biosynthesis over, little is known about how this might be achieved. -

Structural Basis for Phosphatidylinositol-Phosphate Biosynthesis
ARTICLE Received 8 Jun 2015 | Accepted 29 Aug 2015 | Published 16 Oct 2015 DOI: 10.1038/ncomms9505 OPEN Structural basis for phosphatidylinositol-phosphate biosynthesis Oliver B. Clarke1,*, David Tomasek2,*, Carla D. Jorge3,*, Meagan Belcher Dufrisne2, Minah Kim2, Surajit Banerjee4, Kanagalaghatta R. Rajashankar4, Lawrence Shapiro1, Wayne A. Hendrickson1, Helena Santos3 & Filippo Mancia2 Phosphatidylinositol is critical for intracellular signalling and anchoring of carbohydrates and proteins to outer cellular membranes. The defining step in phosphatidylinositol biosynthesis is catalysed by CDP-alcohol phosphotransferases, transmembrane enzymes that use CDP-diacylglycerol as donor substrate for this reaction, and either inositol in eukaryotes or inositol phosphate in prokaryotes as the acceptor alcohol. Here we report the structures of a related enzyme, the phosphatidylinositol-phosphate synthase from Renibacterium salmoninarum, with and without bound CDP-diacylglycerol to 3.6 and 2.5 Å resolution, respectively. These structures reveal the location of the acceptor site, and the molecular determinants of substrate specificity and catalysis. Functional characterization of the 40%-identical ortholog from Mycobacterium tuberculosis, a potential target for the development of novel anti-tuberculosis drugs, supports the proposed mechanism of substrate binding and catalysis. This work therefore provides a structural and functional framework to understand the mechanism of phosphatidylinositol-phosphate biosynthesis. 1 Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA. 2 Department of Physiology and Cellular Biophysics, Columbia University, New York, NY 10032, USA. 3 Biology Division, Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, Avenida da Repu´blica-EAN, 2780-157 Oeiras, Portugal. 4 NE-CATand Department of Chemistry and Chemical Biology, Cornell University, Argonne National Laboratory, Argonne, IL 60439, USA. -

The Mechanisms of Microtubule Catastrophe and Rescue: Implications from Analysis of a Dimer-Scale Computational Model
M BoC | ARTICLE The mechanisms of microtubule catastrophe and rescue: implications from analysis of a dimer-scale computational model Gennady Margolina,b,*,†, Ivan V. Gregorettib,c,*,†, Trevor M. Cickovskid,‡, Chunlei Lia,b, Wei Shia,b,§, Mark S. Albera,b,e, and Holly V. Goodsonb,c aDepartment of Applied and Computational Mathematics and Statistics, bInterdisciplinary Center for the Study of Biocomplexity, cDepartment of Chemistry and Biochemistry, and dDepartment of Computer Science and Engineering, University of Notre Dame, Notre Dame, IN 46556; eDepartment of Medicine, Indiana University School of Medicine, Indianapolis, IN 40202 ABSTRACT Microtubule (MT) dynamic instability is fundamental to many cell functions, but Monitoring Editor its mechanism remains poorly understood, in part because it is difficult to gain information Alexander Mogilner about the dimer-scale events at the MT tip. To address this issue, we used a dimer-scale com- University of California, Davis putational model of MT assembly that is consistent with tubulin structure and biochemistry, Received: Aug 12, 2011 displays dynamic instability, and covers experimentally relevant spans of time. It allows us to Revised: Nov 30, 2011 correlate macroscopic behaviors (dynamic instability parameters) with microscopic structures Accepted: Dec 13, 2011 (tip conformations) and examine protofilament structure as the tip spontaneously progresses through both catastrophe and rescue. The model’s behavior suggests that several commonly held assumptions about MT dynamics should be reconsidered. Moreover, it predicts that short, interprotofilament “cracks” (laterally unbonded regions between protofilaments) exist even at the tips of growing MTs and that rapid fluctuations in the depths of these cracks influ- ence both catastrophe and rescue. -

Glycosyl-Phosphatidylinositol/Inositol Phosphoglycan
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 8016-8019, September 1991 Biochemistry Glycosyl-phosphatidylinositol/inositol phosphoglycan: A signaling system for the low-affinity nerve growth factor receptor (development/inner ear/cochleovestibular ganglion/ant-ostol phosphoglycan antibody) JUAN REPRESA*, MATfAS A. AVILAt, CRISTINA MINERf, FERNANDO GIRALDEZt, GUILLERMO ROMERO§, ROSA CLEMENTEt, JOSE M. MATOt, AND ISABEL VARELA-NIETOt¶ *Departamento Ciencias Morfol6gicas and *Departamento Bioqutmica, Biologfa Molecular y Fisiologfa, Facultad de Medicina, Universidad de Valladolid, 47005 Valladolid, Spain; §Department of Pharmacology, University of Virginia, Charlottesville, VA 22908; and tInstituto de Investigaciones Biomddicas, Consejo Superior de Investigaciones Cientfficas and Departamento Bioqufmica, Universidad Aut6noma de Madrid, Arturo Duperier 4, 28029 Madrid, Spain Communicated by Sidney Udenfriend, June 7, 1991 (received for review April 15, 1991) ABSTRACT Nerve growth factor (NGF) exerts a variety of that IPG would be conserved for some of the developmental actions during embryonic development. At the early stages of actions of insulin and NGF, which could use a common inner ear development, NGF stimulates cell proliferation, an signaling pathway, shared perhaps with other related growth effect mediated through low-affinity receptors. We have stud- factors, to regulate cell growth. The present work provides ied the possibility that the glycosyl-phosphatidylinositol/ further support for the involvement of this glycosyl-PtdIns/ inositol phosphoglycan (glycosyl-Ptdlns/IPG) system is in- IPG pathway in transducing the mitogenic effects of NGF on volved in transmitting this NGF signal. Endogenous glycosyl- the early developing inner ear by showing the following PtdIns was characterized in extracts of cochleovestibular gan- results: (i) the presence of endogenous glycosyl-PtdIns and glia (CVGs) that incorporated [3Hglucosamine, [Hjglactose, IPG, the latter with strong mitogenic activity; (it) the ability [3lH]myristic acid, and PH]palmitic acid. -

EFFECTS of BLOOD FEEDING on the TRANSCRIPTOME of the MALPIGHIAN TUBULES in the ASIAN TIGER MOSQUITO AEDES ALBOPICTUS Thesis
EFFECTS OF BLOOD FEEDING ON THE TRANSCRIPTOME OF THE MALPIGHIAN TUBULES IN THE ASIAN TIGER MOSQUITO AEDES ALBOPICTUS Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master in Science in the Graduate School of The Ohio State University By Carlos J. Esquivel Palma, B.S. Graduate Program in Entomology The Ohio State University 2015 Master’s Examination Committee: Dr. Peter M. Piermarini, Advisor Dr. David L. Denlinger Dr. Andrew P. Michel Copyright by Carlos J. Esquivel Palma 2015 Abstract Mosquitoes are one of the major threats to human health worldwide. They are vectors of protozoans, arboviruses, and filarial nematodes that cause diseases in humans and animals. The Asian tiger mosquito Aedes albopictus is a vector of medically important arboviruses such as dengue fever, chikungunya fever, yellow fever, eastern equine encephalitis, La Crosse encephalitis, and West Nile fever. Control of these diseases often involves control of the mosquito vectors with chemical insecticides. However, the use of a limited number of chemicals with similar modes of actions has led to resistance in several mosquito species. The development of new insecticides with novel modes of action is considered a promising strategy to overcome resistance. The Piermarini lab has recently shown that the renal (Malpighian) tubules of mosquitoes are promising physiological targets to disrupt for killing mosquitoes via a novel mode of action. The Malpighian tubules play a critical role in the acute processing of blood meals, by mediating the rapid excretion of water and ions derived from the ingested blood. However, the physiological roles of Malpighian tubules during the chronic processing of blood meals after the initial diuresis is complete (~1-2 h after feeding) are not known.