Lnositol 1,4,5=Trisphosphate Receptor Binding: Autoradiographic Localization in Rat Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytosolic Phosphorylation of Calnexin Controls Intracellular Ca2ϩ Oscillations Via an Interaction with Serca2b H
Cytosolic Phosphorylation of Calnexin Controls Intracellular Ca2ϩ Oscillations via an Interaction with SERCA2b H. Llewelyn Roderick,* James D. Lechleiter,‡ and Patricia Camacho* *Department of Physiology, and ‡Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, San Antonio, Texas 78229-3900 Abstract. Calreticulin (CRT) and calnexin (CLNX) are of this residue. We also demonstrate by coimmunopre- lectin chaperones that participate in protein folding in cipitation that CLNX physically interacts with the the endoplasmic reticulum (ER). CRT is a soluble ER COOH terminus of SERCA2b and that after dephos- lumenal protein, whereas CLNX is a transmembrane phorylation treatment, this interaction is significantly protein with a cytosolic domain that contains two con- reduced. Together, our results suggest that CRT is sensus motifs for protein kinase (PK) C/proline- uniquely regulated by ER lumenal conditions, whereas directed kinase (PDK) phosphorylation. Using confo- CLNX is, in addition, regulated by the phosphorylation cal Ca2ϩ imaging in Xenopus oocytes, we report here status of its cytosolic domain. The S562 residue in that coexpression of CLNX with sarco endoplasmic CLNX acts as a molecular switch that regulates the reticulum calcium ATPase (SERCA) 2b results in inhi- interaction of the chaperone with SERCA2b, thereby bition of intracellular Ca2ϩ oscillations, suggesting a affecting Ca2ϩ signaling and controlling Ca2ϩ-sensitive functional inhibition of the pump. By site-directed mu- chaperone -
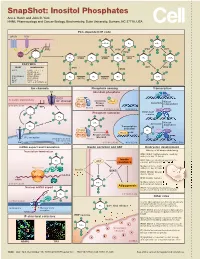
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Neuronal Calcium Sensor-1 Enhancement of Insp3 Receptor Activity Is Inhibited by Therapeutic Levels of Lithium
Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium Christina Schlecker, … , Klara Szigeti-Buck, Barbara E. Ehrlich J Clin Invest. 2006;116(6):1668-1674. https://doi.org/10.1172/JCI22466. Research Article Neuroscience Regulation and dysregulation of intracellular calcium (Ca2+) signaling via the inositol 1,4,5-trisphosphate receptor (InsP3R) has been linked to many cellular processes and pathological conditions. In the present study, addition of neuronal calcium sensor-1 (NCS-1), a high-affinity, low-capacity, calcium-binding protein, to purified InsP3R type 1 (InsP3R1) increased the channel activity in both a calcium-dependent and -independent manner. In intact cells, enhanced expression of NCS-1 resulted in increased intracellular calcium release upon stimulation of the phosphoinositide signaling pathway. To determine whether InsP3R1/NCS-1 interaction could be functionally relevant in bipolar disorders, conditions in which NCS-1 is highly expressed, we tested the effect of lithium, a salt widely used for treatment of bipolar disorders. Lithium inhibited the enhancing effect of NCS-1 on InsP3R1 function, suggesting that InsP3R1/NCS-1 interaction is an essential component of the pathomechanism of bipolar disorder. Find the latest version: https://jci.me/22466/pdf Research article Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium Christina Schlecker,1,2,3 Wolfgang Boehmerle,1,4 Andreas Jeromin,5 Brenda DeGray,1 Anurag Varshney,1 Yogendra Sharma,6 Klara Szigeti-Buck,7 and Barbara E. Ehrlich1,3 1Department of Pharmacology, Yale University, New Haven, Connecticut, USA. 2Department of Neuroscience, University of Magdeburg, Magdeburg, Germany. -

ITPK1 Mediates the Lipid-Independent Synthesis of Inositol Phosphates Controlled by Metabolism
ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism Yann Desfougèresa, Miranda S. C. Wilsona, Debabrata Lahaa, Gregory J. Millerb, and Adolfo Saiardia,1 aMedical Research Council Laboratory for Molecular Cell Biology, University College London, WC1E 6BT London, United Kingdom; and bDepartment of Chemistry, The Catholic University of America, Washington, DC 20064 Edited by Solomon H. Snyder, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved October 25, 2019 (received for review July 3, 2019) Inositol phosphates (IPs) comprise a network of phosphorylated The potential pathways to IP6-7-8 synthesis have a profound molecules that play multiple signaling roles in eukaryotes. IPs impact on how we interpret their signaling roles. If they are synthesis is believed to originate with IP3 generated from PIP2 by synthesized from IP3, their function has evolved in relation to phospholipase C (PLC). Here, we report that in mammalian cells lipid-dependent PLC and/or calcium signaling. This constraint is PLC-generated IPs are rapidly recycled to inositol, and uncover the relieved if IP6 synthesis occurs lipid-independently, originating enzymology behind an alternative “soluble” route to synthesis of directly from inositol, a “soluble” pathway. In yeast, which do not IPs. Inositol tetrakisphosphate 1-kinase 1 (ITPK1)—found in Asgard have IP3-regulated calcium signaling, IP6 synthesis strictly depends archaea, social amoeba, plants, and animals—phosphorylates on the PLC-generated IP3 (Figs. 1A and 2D). The higher com- I(3)P1 originating from glucose-6-phosphate, and I(1)P1 generated plexity of other eukaryotes might allow different pathways for IPs from sphingolipids, to enable synthesis of IP6. -

Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus Fumigatus
Journal of Fungi Article Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus fumigatus Jizhou Li 1, Isabelle Mouyna 1, Christine Henry 1, Frédérique Moyrand 2, Christian Malosse 3, Julia Chamot-Rooke 3, Guilhem Janbon 2, Jean-Paul Latgé 1 and Thierry Fontaine 1,* 1 Unité des Aspergillus, 25 rue du Docteur Roux, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (J.L.); [email protected] (I.M.); [email protected] (C.H.); [email protected] (J.-P.L.) 2 Unité de Biologie des ARN des Pathogènes Fongiques, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (F.M.); [email protected] (G.J.) 3 Unité de Spectrométrie de Masse pour la Biologie, Institut Pasteur, CNRS USR 2000, 28 rue du Docteur Roux, 75015 Paris, France; [email protected] (C.M.); [email protected] (J.C.-R.) * Correspondence: [email protected]; Tel.: +33-145-688-358 Received: 8 December 2017; Accepted: 19 January 2018; Published: 2 Febuary 2018 Abstract: Glycosylphosphatidylinositols (GPIs) are lipid anchors allowing the exposure of proteins at the outer layer of the plasma membrane. In fungi, a number of GPI-anchored proteins (GPI-APs) are involved in the remodeling of the cell wall polymers. GPIs follow a specific biosynthetic pathway in the endoplasmic reticulum. After the transfer of the protein onto the GPI-anchor, a lipid remodeling occurs to substitute the diacylglycerol moiety by a ceramide. In addition to GPI-APs, A. fumigatus produces a GPI-anchored polysaccharide, the galactomannan (GM), that remains unique in the fungal kingdom. -

Calcineurin Associated with the Lnositol 1,4,5=Trisphosphate Receptor-FKBPI 2 Complex Modulates Ca*+ Flux
Cell, Vol. 83,463-472, November 3, 1995, Copyright 0 1995 by Cell Press Calcineurin Associated with the lnositol 1,4,5=Trisphosphate Receptor-FKBPI 2 Complex Modulates Ca*+ Flux Andrew M. Cameron,* Joseph P. Steiner,* such as the transcription factor NFAT (for nuclear factor A. Jane Roskams, l Siraj M. Ali, * Gabriele V. Ronnett,? of activated T cells) (Liu et al., 1991; O’Keefe et al., 1992; and Solomon H. Snyder**5 Clipstone and Crabtree, 1992; for review of calcineurin, *Department of Neuroscience see Klee et al., 1988). In its phosphorylated state, NFAT tDepartment of Neurology cannot translocate to the nucleus, where it regulates ex- $Department of Pharmacology and Molecular Sciences pression of genes critical for T cell activation such as in- SDepartment of Psychiatry and Behavioral Sciences terleukin-2 (IL-2). Rapamycin acts differently than cyclo- Johns Hopkins University School of Medicine sporin A and FK506, as it blocks the actions of IL-2 rather Baltimore, Maryland 21205 than its synthesis (Bierer et al., 1990). Rapamycin binds with high affinity to FKBP12 and inhibits its rotamase activ- ity, but the rapamycin-FKBP12 complex does not interact Summary with calcineurin. Rather, this immunosuppressant-immu- nophilin complex binds to a recently identified target pro- The immunosuppressant drug FK506 binds to the im- tein designated RAFT (for rapamycin and FKBP12 target) munophilin protein FKBPl2 and inhibits its prolyl iso- or FRAP (for FKBP-rapamycin-associated protein) (Sa- meraseactivity. Immunosuppresiveactions, however, batini et al., 1994; Brown et al., 1994). are mediated via an FK506-FKBP12 inhibition of the Marks and colleagues have observed an association Ca*+-activated phosphatase calcineurin. -

Dephosphorylation of 2,3-Bisphosphoglycerate by MIPP Expands the Regulatory Capacity of the Rapoport–Luebering Glycolytic Shunt
Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport–Luebering glycolytic shunt Jaiesoon Cho*†, Jason S. King‡§, Xun Qian*, Adrian J. Harwood‡, and Stephen B. Shears*¶ *Laboratory of Signal Transduction, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Social Services, P.O. Box 12233, Research Triangle Park, NC 27709; and ‡Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3US, United Kingdom Edited by Helen M. Ranney, University of California at San Diego, La Jolla, CA, and approved March 6, 2008 (received for review November 21, 2007) The Rapoport–Luebering glycolytic bypass comprises evolutionar- majority of mammals. By preferentially binding to deoxyhemo- ily conserved reactions that generate and dephosphorylate 2,3- globin, 2,3-BPG facilitates oxygen release from the erythrocyte bisphosphoglycerate (2,3-BPG). For >30 years, these reactions have to the surrounding tissues (6, 7). Thus, the regulation of eryth- been considered the responsibility of a single enzyme, the 2,3-BPG rocyte 2,3-BPG levels is key to efficiently meeting tissue oxygen synthase/2-phosphatase (BPGM). Here, we show that Dictyosteli- demands while also providing an important physiological adap- um, birds, and mammals contain an additional 2,3-BPG phospha- tation to oxygen deprivation (8), including that which occurs at tase that, unlike BPGM, removes the 3-phosphate. This discovery high altitude (9) or during postoperative anemia (10). reveals that the glycolytic pathway can bypass the formation of Despite the importance of carefully regulating 2,3-BPG turn- 3-phosphoglycerate, which is a precursor for serine biosynthesis over, little is known about how this might be achieved. -

Structural Basis for Phosphatidylinositol-Phosphate Biosynthesis
ARTICLE Received 8 Jun 2015 | Accepted 29 Aug 2015 | Published 16 Oct 2015 DOI: 10.1038/ncomms9505 OPEN Structural basis for phosphatidylinositol-phosphate biosynthesis Oliver B. Clarke1,*, David Tomasek2,*, Carla D. Jorge3,*, Meagan Belcher Dufrisne2, Minah Kim2, Surajit Banerjee4, Kanagalaghatta R. Rajashankar4, Lawrence Shapiro1, Wayne A. Hendrickson1, Helena Santos3 & Filippo Mancia2 Phosphatidylinositol is critical for intracellular signalling and anchoring of carbohydrates and proteins to outer cellular membranes. The defining step in phosphatidylinositol biosynthesis is catalysed by CDP-alcohol phosphotransferases, transmembrane enzymes that use CDP-diacylglycerol as donor substrate for this reaction, and either inositol in eukaryotes or inositol phosphate in prokaryotes as the acceptor alcohol. Here we report the structures of a related enzyme, the phosphatidylinositol-phosphate synthase from Renibacterium salmoninarum, with and without bound CDP-diacylglycerol to 3.6 and 2.5 Å resolution, respectively. These structures reveal the location of the acceptor site, and the molecular determinants of substrate specificity and catalysis. Functional characterization of the 40%-identical ortholog from Mycobacterium tuberculosis, a potential target for the development of novel anti-tuberculosis drugs, supports the proposed mechanism of substrate binding and catalysis. This work therefore provides a structural and functional framework to understand the mechanism of phosphatidylinositol-phosphate biosynthesis. 1 Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA. 2 Department of Physiology and Cellular Biophysics, Columbia University, New York, NY 10032, USA. 3 Biology Division, Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, Avenida da Repu´blica-EAN, 2780-157 Oeiras, Portugal. 4 NE-CATand Department of Chemistry and Chemical Biology, Cornell University, Argonne National Laboratory, Argonne, IL 60439, USA. -

Glycosyl-Phosphatidylinositol/Inositol Phosphoglycan
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 8016-8019, September 1991 Biochemistry Glycosyl-phosphatidylinositol/inositol phosphoglycan: A signaling system for the low-affinity nerve growth factor receptor (development/inner ear/cochleovestibular ganglion/ant-ostol phosphoglycan antibody) JUAN REPRESA*, MATfAS A. AVILAt, CRISTINA MINERf, FERNANDO GIRALDEZt, GUILLERMO ROMERO§, ROSA CLEMENTEt, JOSE M. MATOt, AND ISABEL VARELA-NIETOt¶ *Departamento Ciencias Morfol6gicas and *Departamento Bioqutmica, Biologfa Molecular y Fisiologfa, Facultad de Medicina, Universidad de Valladolid, 47005 Valladolid, Spain; §Department of Pharmacology, University of Virginia, Charlottesville, VA 22908; and tInstituto de Investigaciones Biomddicas, Consejo Superior de Investigaciones Cientfficas and Departamento Bioqufmica, Universidad Aut6noma de Madrid, Arturo Duperier 4, 28029 Madrid, Spain Communicated by Sidney Udenfriend, June 7, 1991 (received for review April 15, 1991) ABSTRACT Nerve growth factor (NGF) exerts a variety of that IPG would be conserved for some of the developmental actions during embryonic development. At the early stages of actions of insulin and NGF, which could use a common inner ear development, NGF stimulates cell proliferation, an signaling pathway, shared perhaps with other related growth effect mediated through low-affinity receptors. We have stud- factors, to regulate cell growth. The present work provides ied the possibility that the glycosyl-phosphatidylinositol/ further support for the involvement of this glycosyl-PtdIns/ inositol phosphoglycan (glycosyl-Ptdlns/IPG) system is in- IPG pathway in transducing the mitogenic effects of NGF on volved in transmitting this NGF signal. Endogenous glycosyl- the early developing inner ear by showing the following PtdIns was characterized in extracts of cochleovestibular gan- results: (i) the presence of endogenous glycosyl-PtdIns and glia (CVGs) that incorporated [3Hglucosamine, [Hjglactose, IPG, the latter with strong mitogenic activity; (it) the ability [3lH]myristic acid, and PH]palmitic acid. -

CD95 Fas Induces Cleavage of the Grpl Gads Adaptor And
CD95͞Fas induces cleavage of the GrpL͞Gads adaptor and desensitization of antigen receptor signaling Thomas M. Yankee*†, Kevin E. Draves*, Maria K. Ewings*, Edward A. Clark*‡§, and Jonathan D. Graves‡ Departments of *Microbiology and ‡Immunology, and §Regional Primate Research Center, University of Washington, Seattle, WA 98195 Communicated by Edwin G. Krebs, University of Washington School of Medicine, Seattle, WA, April 2, 2001 (received for review February 5, 2001) The balance between cell survival and cell death is critical for SLP-76 via its C-terminal SH3 domain and inducibly binds LAT normal lymphoid development. This balance is maintained by via its SH2 domain (5, 8–10). Thus, GrpL plays a key role in signals through lymphocyte antigen receptors and death recep- calcium mobilization and in the activation of the transcription tors such as CD95͞Fas. In some cells, ligating the B cell antigen factor NF-AT. Here we show that CD95 ligation leads to the receptor can protect the cell from apoptosis induced by CD95. cleavage of GrpL so that it cannot bind SLP-76. The truncated Here we report that ligation of CD95 inhibits antigen receptor- form of GrpL inhibits antigen receptor-mediated NF-AT mediated signaling. Pretreating CD40-stimulated tonsillar B cells signaling. with anti-CD95 abolished B cell antigen receptor-mediated cal- cium mobilization. Furthermore, CD95 ligation led to the Materials and Methods caspase-dependent inhibition of antigen receptor-induced cal- Cells and Antibodies. The human B cell lines MP-1 and BJAB and cium mobilization and to the activation of mitogen-activated the human T cell line Jurkat E6.1 were grown in RPMI medium protein kinase pathways in B and T cell lines. -

Phosphatidylinositol 4,5-Bisphosphate Modifies Tubulin
The Journal of Neuroscience, March 1, 2002, 22(5):1668–1678 Phosphatidylinositol 4,5-Bisphosphate Modifies Tubulin  Participation in Phospholipase C 1 Signaling Juliana S. Popova,1 Arin K. Greene,1 Jia Wang,1 and Mark M. Rasenick1,2 Departments of 1Physiology and Biophysics and 2Psychiatry, University of Illinois at Chicago, College of Medicine, Chicago, Illinois 60612-7342 Tubulin forms the microtubule and regulates certain G-protein- the plasma membrane was demonstrated with confocal laser mediated signaling pathways. Both functions rely on the GTP- immunofluorescence microscopy. Although tubulin bound to ␣ ␣  binding properties of tubulin. Signal transduction through G q- both G q and PLC 1 , PIP2 facilitated the interaction between    ␣ regulated phospholipase C 1 (PLC 1 ) is activated by tubulin tubulin and PLC 1 but not that between tubulin and G q. ␣ ␣  through a direct transfer of GTP from tubulin to G q. However, However, PIP2 did augment formation of tubulin–G q–PLC 1   at high tubulin concentrations, inhibition of PLC 1 is observed. complexes. Subsequent to potentiating PLC 1 activation, sus-  This report demonstrates that tubulin inhibits PLC 1 by binding tained agonist-independent membrane binding of tubulin   ␣ the PLC 1 substrate phosphatidylinositol 4,5-bisphosphate at PIP2- and PLC 1-rich sites appeared to inhibit G q coupling  (PIP2 ). Tubulin binding of PIP2 was specific, because PIP2 but to PLC 1. Furthermore, colchicine increased membrane-  not phosphatidylinositol 3,4,5-trisphosphate, phosphatidylino- associated tubulin and also inhibited PLC 1 activity in SK- sitol 3-phosphate, phosphatidylinositol, phosphatidylcholine, N-SH cells. Thus, tubulin, depending on local membrane con- phosphatidylethanolamine, or inositol 1,4,5-trisphosphate in- centration, may serve as a positive or negative regulator of hibited microtubule assembly. -

Regulation of Autophagy by the Inositol Trisphosphate Receptor
Cell Death and Differentiation (2007) 14, 1029–1039 & 2007 Nature Publishing Group All rights reserved 1350-9047/07 $30.00 www.nature.com/cdd Regulation of autophagy by the inositol trisphosphate receptor A Criollo1,2,3,4, MC Maiuri1,2,5, E Tasdemir1,2,3, I Vitale1,2,3, AA Fiebig6, D Andrews6, J Molgo´ 7,JDı´az4, S Lavandero4, F Harper8, G Pierron8, D di Stefano9, R Rizzuto9, G Szabadkai9 and G Kroemer*,1,2,3 The reduction of intracellular 1,4,5-inositol trisphosphate (IP3) levels stimulates autophagy, whereas the enhancement of IP3 levels inhibits autophagy induced by nutrient depletion. Here, we show that knockdown of the IP3 receptor (IP3R) with small interfering RNAs and pharmacological IP3R blockade is a strong stimulus for the induction of autophagy. The IP3R is known to reside in the membranes of the endoplasmic reticulum (ER) as well as within ER–mitochondrial contact sites, and IP3R blockade triggered the autophagy of both ER and mitochondria, as exactly observed in starvation-induced autophagy. ER stressors such as tunicamycin and thapsigargin also induced autophagy of ER and, to less extent, of mitochondria. Autophagy triggered by starvation or IP3R blockade was inhibited by Bcl-2 and Bcl-XL specifically targeted to ER but not Bcl-2 or Bcl-XL proteins targeted to mitochondria. In contrast, ER stress-induced autophagy was not inhibited by Bcl-2 and Bcl-XL. Autophagy promoted by IP3R inhibition could not be attributed to a modulation of steady-state Ca2 þ levels in the ER or in the cytosol, yet involved the obligate contribution of Beclin-1, autophagy-related gene (Atg)5, Atg10, Atg12 and hVps34.