Dephosphorylation of 2,3-Bisphosphoglycerate by MIPP Expands the Regulatory Capacity of the Rapoport–Luebering Glycolytic Shunt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
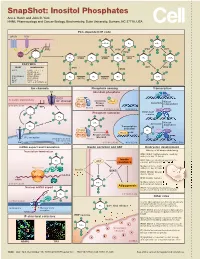
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Annotation of Glycolysis and Gluconeogenesis Pathways
A metabolic insight into the Asian citrus psyllid: Annotation of glycolysis and gluconeogenesis pathways Blessy Tamayo, Kyle Kercher, Tom D’Elia, Helen Wiersma-Koch, Surya Saha, Teresa Shippy, Susan J. Brown, and Prashant Hosmani Introduction Glycolysis, as in other animals, is the major metabolic pathway that insects use to extract energy from carbohydrates [1]. This process consists of ten reactions that convert one molecule of glucose into two molecules of pyruvate within the cytosol, generating a net gain of two molecules of ATP. Simple carbohydrates are acquired through the insect diet and processed through glycolysis and the central carbohydrate metabolic steps. Comparative genomic analysis of Apis mellifera, Drosophila melanogaster, and Anopheles gambiae has revealed the presence of genes for metabolic pathways that support dietary habits dependent on sugar-rich substrates ([2]; [3]; [4]; [5]). In addition to processing sugars for energy, glycolysis also serves as a central pathway that serves as both a source of important precursors and destination for key intermediates from many metabolic pathways. Gluconeogenesis is the process through which glucose is synthesized from non- carbohydrate substrates, and is closely associated with glycolysis. Eleven enzymatic reactions occur during gluconeogenesis. Eight of the enzymes involved in the steps also catalyze the reverse reactions in glycolysis and the three remaining enzymes are specific to gluconeogenesis. 1 Gluconeogenesis generated carbohydrates are required as substrate for anaerobic glycolysis, synthesis of chitin, glycoproteins, polyols and glycoside detoxication products [1]. Gluconeogenesis is essential in insects to maintain sugar homeostasis and serves as the initial process towards the generation of glucose disaccharide trehalose, which is the main circulating sugar in the insect hemolymph ([6]; [7]). -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Phosphoglycerate Mutase Deficiency
Phosphoglycerate mutase deficiency Description Phosphoglycerate mutase deficiency is a disorder that primarily affects muscles used for movement (skeletal muscles). Beginning in childhood or adolescence, affected individuals experience muscle aches or cramping following strenuous physical activity. Some people with this condition also have recurrent episodes of myoglobinuria. Myoglobinuria occurs when muscle tissue breaks down abnormally and releases a protein called myoglobin, which is processed by the kidneys and released in the urine. If untreated, myoglobinuria can lead to kidney failure. In some cases of phosphoglycerate mutase deficiency, microscopic tube-shaped structures called tubular aggregates are seen in muscle fibers. It is unclear how tubular aggregates are associated with the signs and symptoms of the disorder. Frequency Phosphoglycerate mutase deficiency is a rare condition; about 15 affected people have been reported in the medical literature. Most affected individuals have been African American. Causes Phosphoglycerate mutase deficiency is caused by mutations in the PGAM2 gene. This gene provides instructions for making an enzyme called phosphoglycerate mutase, which is involved in a critical energy-producing process in cells known as glycolysis. During glycolysis, the simple sugar glucose is broken down to produce energy. The version of phosphoglycerate mutase produced from the PGAM2 gene is found primarily in skeletal muscle cells. Mutations in the PGAM2 gene greatly reduce the activity of phosphoglycerate mutase, which disrupts energy production in these cells. This defect underlies the muscle cramping and myoglobinuria that occur after strenuous exercise in affected individuals. Learn more about the gene associated with Phosphoglycerate mutase deficiency • PGAM2 Reprinted from MedlinePlus Genetics (https://medlineplus.gov/genetics/) 1 I nheritance This condition is inherited in an autosomal recessive pattern, which means both copies of the PGAM2 gene in each cell have mutations. -

2 Points Chem 465 Biochemistry II Test 1 Spring 2017 Multiple Choice
Name: 2 points Chem 465 Biochemistry II Test 1 Spring 2017 Multiple choice (4 points apiece): 1. Which of the following statements about the oxidative decarboxylation of pyruvate in aerobic conditions in animal cells is correct? A) One of the products of the reactions of the pyruvate dehydrogenase complex is a thioester of acetate. B) The methyl (-CH3) group is eliminated as CO2. C) The process occurs in the cytosolic compartment of the cell. D) The pyruvate dehydrogenase complex uses all of the following as cofactors: NAD+, lipoic acid, pyridoxal phosphate (PLP), and FAD. E) The reaction is so important to energy production that pyruvate dehydrogenase operates at full speed under all conditions. 2. In comparison with the resting state, actively contracting human muscle tissue has a: A) higher concentration of ATP. B) higher rate of lactate formation. C) lower consumption of glucose. D) lower rate of consumption of oxygen E) lower ratio of NADH to NAD+. 3.The metabolic function of the pentose phosphate pathway is: A) act as a source of ADP biosynthesis. B) generate NADPH and pentoses for the biosynthesis of fatty acids and nucleic acids. C) participate in oxidation-reduction reactions during the formation of H2O. D) provide intermediates for the citric acid cycle. E) synthesize phosphorus pentoxide. 4. Gluconeogenesis must use "bypass reactions" to circumvent three reactions in the glycolytic pathway that are highly exergonic and essentially irreversible. Reactions carried out by which three of the enzymes listed must be bypassed in the gluconeogenic pathway? 1) Hexokinase 2) Phosphoglycerate kinase 3) Phosphofructokinase-1 4) Pyruvate kinase 5) Triosephosphate isomerase A) 1, 2, 3 B) 1, 2, 4 C) 1, 4, 5 D) 1, 3, 4 E) 2, 3, 4 5. -

MITOCW | Watch?V=Vl E7ik Vbs
MITOCW | watch?v=vL_E7Ik_vBs The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu. DR. BOGDAN Hello, and welcome to 5.07 Biochemistry online. I'm Dr. Bogdan Fedeles. Let's metabolize FEDELES: some problems. Today we're discussing problem 2 of problem set 6. Here we're going to explore in more detail the mechanism of phosphoglycerate mutase, which is the eighth enzyme in glycolysis. It's the enzyme that catalyzes the conversion of 3-phosphoglycerate to 2-phosphoglycerate. Generally speaking, mutases are enzymes that catalyze the shift of a functional group between two similar positions of a molecule. In the case of phosphoglycerate mutase, this enzyme catalyzes the transfer of the phosphate group from the 3 position of glycerate to the 2 position of glycerate. In 5.07, you will encounter several mutases. Similar to phosphoglycerate mutase, there is a bisphosphoglycerate mutase, which converts 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate. Now, this reaction is very important when it happens in the red blood cells. Another mutase you will encounter is in the glycogen breakdown pathway. It's called phosphoglucomutase and converts glucose 1-phosphate to glucose 6-phosphate. Now finally, the most intriguing of them all is the methylmalonyl-coa mutase, which is a fascinating enzyme that converts methylmalonyl-coA to succinyl-coA. In this reaction, it rearranges this carbon skeleton of the molecule, and it requires adenosylcobalamin, which is a co-factor derived from vitamin B12. -

Mechanisms of Interaction Between Haemophilus Parainfluenzae and Streptococcus Mitis
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2021 MECHANISMS OF INTERACTION BETWEEN HAEMOPHILUS PARAINFLUENZAE AND STREPTOCOCCUS MITIS Dasith Perera Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss MECHANISMS OF INTERACTION BETWEEN HAEMOPHILUS PARAINFLUENZAE AND STREPTOCOCCUS MITIS BY DASITH PERERA A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN CELL & MOLECULAR BIOLOGY UNIVERSITY OF RHODE ISLAND 2021 DOCTOR OF PHILOSOPHY DISSERTATION OF DASITH PERERA APPROVED: Thesis Committee: Matthew Ramsey, Major professor David Nelson David Rowley Brenton DeBoef DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2021 ABSTRACT The human oral cavity is a complex polymicrobial environment, home to an array of microbes that play roles in health and disease. Oral bacteria have been shown to cause an array of systemic diseases and are particularly concerning to type II diabetics (T2D) with numerous predispositions that exacerbate bacterial infection. In this dissertation, we investigated the serum of healthy subjects and T2D subjects to determine whether we see greater translocation of oral bacteria into the bloodstream of T2D indiviDuals. We didn’t observe any significant enrichment of oral taxa, however we detected the presence of an emerging pathogen, Acinetobacter baumannii that is also associated with impaired inflammation in T2D. While some are associated with disease, many oral taxa are important in the pre- vention of disease. In this dissertation we investigated the interactions between two abundant health-associated commensal microbes, Haemophilus parainfluenzae and Streptococcus mitis. We demonstrated that H. parainfluenzae typically exists adjacent to Mitis group streptococci in vivo in healthy subjects. -

Supplementary Informations SI2. Supplementary Table 1
Supplementary Informations SI2. Supplementary Table 1. M9, soil, and rhizosphere media composition. LB in Compound Name Exchange Reaction LB in soil LBin M9 rhizosphere H2O EX_cpd00001_e0 -15 -15 -10 O2 EX_cpd00007_e0 -15 -15 -10 Phosphate EX_cpd00009_e0 -15 -15 -10 CO2 EX_cpd00011_e0 -15 -15 0 Ammonia EX_cpd00013_e0 -7.5 -7.5 -10 L-glutamate EX_cpd00023_e0 0 -0.0283302 0 D-glucose EX_cpd00027_e0 -0.61972444 -0.04098397 0 Mn2 EX_cpd00030_e0 -15 -15 -10 Glycine EX_cpd00033_e0 -0.0068175 -0.00693094 0 Zn2 EX_cpd00034_e0 -15 -15 -10 L-alanine EX_cpd00035_e0 -0.02780553 -0.00823049 0 Succinate EX_cpd00036_e0 -0.0056245 -0.12240603 0 L-lysine EX_cpd00039_e0 0 -10 0 L-aspartate EX_cpd00041_e0 0 -0.03205557 0 Sulfate EX_cpd00048_e0 -15 -15 -10 L-arginine EX_cpd00051_e0 -0.0068175 -0.00948672 0 L-serine EX_cpd00054_e0 0 -0.01004986 0 Cu2+ EX_cpd00058_e0 -15 -15 -10 Ca2+ EX_cpd00063_e0 -15 -100 -10 L-ornithine EX_cpd00064_e0 -0.0068175 -0.00831712 0 H+ EX_cpd00067_e0 -15 -15 -10 L-tyrosine EX_cpd00069_e0 -0.0068175 -0.00233919 0 Sucrose EX_cpd00076_e0 0 -0.02049199 0 L-cysteine EX_cpd00084_e0 -0.0068175 0 0 Cl- EX_cpd00099_e0 -15 -15 -10 Glycerol EX_cpd00100_e0 0 0 -10 Biotin EX_cpd00104_e0 -15 -15 0 D-ribose EX_cpd00105_e0 -0.01862144 0 0 L-leucine EX_cpd00107_e0 -0.03596182 -0.00303228 0 D-galactose EX_cpd00108_e0 -0.25290619 -0.18317325 0 L-histidine EX_cpd00119_e0 -0.0068175 -0.00506825 0 L-proline EX_cpd00129_e0 -0.01102953 0 0 L-malate EX_cpd00130_e0 -0.03649016 -0.79413596 0 D-mannose EX_cpd00138_e0 -0.2540567 -0.05436649 0 Co2 EX_cpd00149_e0 -

ITPK1 Mediates the Lipid-Independent Synthesis of Inositol Phosphates Controlled by Metabolism
ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism Yann Desfougèresa, Miranda S. C. Wilsona, Debabrata Lahaa, Gregory J. Millerb, and Adolfo Saiardia,1 aMedical Research Council Laboratory for Molecular Cell Biology, University College London, WC1E 6BT London, United Kingdom; and bDepartment of Chemistry, The Catholic University of America, Washington, DC 20064 Edited by Solomon H. Snyder, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved October 25, 2019 (received for review July 3, 2019) Inositol phosphates (IPs) comprise a network of phosphorylated The potential pathways to IP6-7-8 synthesis have a profound molecules that play multiple signaling roles in eukaryotes. IPs impact on how we interpret their signaling roles. If they are synthesis is believed to originate with IP3 generated from PIP2 by synthesized from IP3, their function has evolved in relation to phospholipase C (PLC). Here, we report that in mammalian cells lipid-dependent PLC and/or calcium signaling. This constraint is PLC-generated IPs are rapidly recycled to inositol, and uncover the relieved if IP6 synthesis occurs lipid-independently, originating enzymology behind an alternative “soluble” route to synthesis of directly from inositol, a “soluble” pathway. In yeast, which do not IPs. Inositol tetrakisphosphate 1-kinase 1 (ITPK1)—found in Asgard have IP3-regulated calcium signaling, IP6 synthesis strictly depends archaea, social amoeba, plants, and animals—phosphorylates on the PLC-generated IP3 (Figs. 1A and 2D). The higher com- I(3)P1 originating from glucose-6-phosphate, and I(1)P1 generated plexity of other eukaryotes might allow different pathways for IPs from sphingolipids, to enable synthesis of IP6. -

Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus Fumigatus
Journal of Fungi Article Glycosylphosphatidylinositol Anchors from Galactomannan and GPI-Anchored Protein Are Synthesized by Distinct Pathways in Aspergillus fumigatus Jizhou Li 1, Isabelle Mouyna 1, Christine Henry 1, Frédérique Moyrand 2, Christian Malosse 3, Julia Chamot-Rooke 3, Guilhem Janbon 2, Jean-Paul Latgé 1 and Thierry Fontaine 1,* 1 Unité des Aspergillus, 25 rue du Docteur Roux, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (J.L.); [email protected] (I.M.); [email protected] (C.H.); [email protected] (J.-P.L.) 2 Unité de Biologie des ARN des Pathogènes Fongiques, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; [email protected] (F.M.); [email protected] (G.J.) 3 Unité de Spectrométrie de Masse pour la Biologie, Institut Pasteur, CNRS USR 2000, 28 rue du Docteur Roux, 75015 Paris, France; [email protected] (C.M.); [email protected] (J.C.-R.) * Correspondence: [email protected]; Tel.: +33-145-688-358 Received: 8 December 2017; Accepted: 19 January 2018; Published: 2 Febuary 2018 Abstract: Glycosylphosphatidylinositols (GPIs) are lipid anchors allowing the exposure of proteins at the outer layer of the plasma membrane. In fungi, a number of GPI-anchored proteins (GPI-APs) are involved in the remodeling of the cell wall polymers. GPIs follow a specific biosynthetic pathway in the endoplasmic reticulum. After the transfer of the protein onto the GPI-anchor, a lipid remodeling occurs to substitute the diacylglycerol moiety by a ceramide. In addition to GPI-APs, A. fumigatus produces a GPI-anchored polysaccharide, the galactomannan (GM), that remains unique in the fungal kingdom. -

Multiple Enzyme Purifications from Muscle Extracts by Using Affinity-Elution- Chromatographic Procedures
Biochem. J. (1977) 161, 265-277 265 Printed in Great Britain Multiple Enzyme Purifications from Muscle Extracts by using Affinity-Elution- Chromatographic Procedures By ROBERT K. SCOPES Department ofBiochemistry, La Trobe University, Bundoora, Vic. 3083, Australia (Received 20 July 1976) 1. Starting with (NH4)2SO4 fractions of muscle extracts, procedures for purifying four to six separate enzymes from each fraction by using affinity-elution-chromatographic techniques are described. 2. Schemes for purifying 12 separate enzymes from rabbit muscle, and eight from chicken muscle extracts, are included. In nearly all cases the overall procedure involves three steps: the initial (NH4)2SO4 fractionation, the ion- exchange chromatography with affinity elution of the enzyme, and gel filtration. The specific activities of the enzymes so purified are comparable with the highest values in the literature. 3. The five schemes described include illustrations of affinity elution of the separate enzymes at different pH values, at different ionic strengths and in combination with conventional gradient elution. They also include stepwise adsorption on columns at different pH values. 4. Separation of two electrophoretically differing forms of phospho- glycerate kinase was achieved by gradient affinity elution from CM-cellulose. The lower-pI form was eluted by a lower concentration of substrate than the higher-pI form. Methods for purifying individual glycolytic and of extract (approx. 350g of muscle), and the columns related enzymes from rabbit muscle by using affinity- used were always 16cm2 cross-section and close to elution techniques were described in the preceding 5cm tall unless otherwise indicated. The buffers used paper (Scopes, 1977). Although most investigators in chromatography were 10mM of base (Tris, KOH, are interested in only one or perhaps two different NaOH) adjusted to the required pH with a-picolinic enzymes, it may be desirable to purify others to use, acid, Mes,* Mops or Tricine as appropriate. -

Structural Basis for Phosphatidylinositol-Phosphate Biosynthesis
ARTICLE Received 8 Jun 2015 | Accepted 29 Aug 2015 | Published 16 Oct 2015 DOI: 10.1038/ncomms9505 OPEN Structural basis for phosphatidylinositol-phosphate biosynthesis Oliver B. Clarke1,*, David Tomasek2,*, Carla D. Jorge3,*, Meagan Belcher Dufrisne2, Minah Kim2, Surajit Banerjee4, Kanagalaghatta R. Rajashankar4, Lawrence Shapiro1, Wayne A. Hendrickson1, Helena Santos3 & Filippo Mancia2 Phosphatidylinositol is critical for intracellular signalling and anchoring of carbohydrates and proteins to outer cellular membranes. The defining step in phosphatidylinositol biosynthesis is catalysed by CDP-alcohol phosphotransferases, transmembrane enzymes that use CDP-diacylglycerol as donor substrate for this reaction, and either inositol in eukaryotes or inositol phosphate in prokaryotes as the acceptor alcohol. Here we report the structures of a related enzyme, the phosphatidylinositol-phosphate synthase from Renibacterium salmoninarum, with and without bound CDP-diacylglycerol to 3.6 and 2.5 Å resolution, respectively. These structures reveal the location of the acceptor site, and the molecular determinants of substrate specificity and catalysis. Functional characterization of the 40%-identical ortholog from Mycobacterium tuberculosis, a potential target for the development of novel anti-tuberculosis drugs, supports the proposed mechanism of substrate binding and catalysis. This work therefore provides a structural and functional framework to understand the mechanism of phosphatidylinositol-phosphate biosynthesis. 1 Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA. 2 Department of Physiology and Cellular Biophysics, Columbia University, New York, NY 10032, USA. 3 Biology Division, Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, Avenida da Repu´blica-EAN, 2780-157 Oeiras, Portugal. 4 NE-CATand Department of Chemistry and Chemical Biology, Cornell University, Argonne National Laboratory, Argonne, IL 60439, USA.