Role of Inositols and Inositol Phosphates in Energy Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inositol Safety: Clinical Evidences
European Review for Medical and Pharmacological Sciences 2011; 15: 931-936 Inositol safety: clinical evidences G. CARLOMAGNO, V. UNFER AGUNCO Obstetrics & Gynecology Center, Rome (Italy) Abstract. – Myo-inositol is a six carbon ent required by the human cells for the growth cyclitol that contains five equatorial and one axi- and survival in the culture. In humans and other al hydroxyl groups. Myo-inositol has been classi- species, Myo-inositol can be converted to either fied as an insulin sensitizing agent and it is L- or D-chiro-inositol by epimerases. Early stud- commonly used in the treatment of the Polycys- tic Ovary Syndrome (PCOS). However, despite ies showed that inositol urinary clearance was al- its wide clinical use, there is still scarce informa- tered in type 2 diabetes patients, the next step tion on the myo-inositol safety and/or side ef- was to link impaired inositol clearance with in- fects. The aim of the present review was to sum- sulin resistance (for a review see1). Because of marize and discuss available data on the myo-in- these properties, inositol have been classified as ositol safety both in non-clinical and clinical set- “insulin sensitizing agent”2. tings. The main outcome was that only the highest In the recent years, inositol has found more dose of myo-inositol (12 g/day) induced mild and more space in the reproductive clinical prac- gastrointestinal side effects such as nausea, fla- tice3-6. Indeed, since the main therapy for Poly- tus and diarrhea. The severity of side effects did cystic Ovary Syndrome (PCOS) is the use of in- not increase with the dosage. -

Phosphorylation of Synaptojanin Differentially Regulates Endocytosis of Functionally Distinct Synaptic Vesicle Pools
8882 • The Journal of Neuroscience, August 24, 2016 • 36(34):8882–8894 Cellular/Molecular Phosphorylation of Synaptojanin Differentially Regulates Endocytosis of Functionally Distinct Synaptic Vesicle Pools X Junhua Geng,1* Liping Wang,1,2* Joo Yeun Lee,1,4 XChun-Kan Chen,1 and Karen T. Chang1,3,4 1Zilkha Neurogenetic Institute, 2Department of Biochemistry and Molecular Biology, and 3Department of Cell and Neurobiology, Keck School of Medicine, University of Southern California, Los Angeles, California 90089, and 4Neuroscience Graduate Program, University of Southern California, Los Angeles, California 90089 The rapid replenishment of synaptic vesicles through endocytosis is crucial for sustaining synaptic transmission during intense neuronal activity. Synaptojanin (Synj), a phosphoinositide phosphatase, is known to play an important role in vesicle recycling by promoting the uncoating of clathrin following synaptic vesicle uptake. Synj has been shown to be a substrate of the minibrain (Mnb) kinase, a fly homolog of the dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A); however, the functional impacts of Synj phosphorylation by Mnb are not well understood. Here we identify that Mnb phosphorylates Synj at S1029 in Drosophila. We find that phosphorylation of Synj at S1029 enhances Synj phosphatase activity, alters interaction between Synj and endophilin, and promotes efficient endocytosis of the active cycling vesicle pool (also referred to as exo-endo cycling pool) at the expense of reserve pool vesicle endocytosis. Dephosphorylated Synj, on the other hand, is deficient in the endocytosis of the active recycling pool vesicles but maintains reserve pool vesicle endocytosis to restore total vesicle pool size and sustain synaptic transmission. Together, our findings reveal a novel role for Synj in modulating reserve pool vesicle endocytosis and further indicate that dynamic phosphorylation and dephosphorylation of Synj differentially maintain endocytosis of distinct functional synaptic vesicle pools. -
Revisions to USP 31–NF 26, Second Supplement
Revisions to USP 31–NF 26, Second Supplement (published May 2008) Published April 2008 General Chapters Monographs:A–C D–N O–S T–Z Monograph Title Section Head Scientific Liaison DANTROLENE SODIUM Dissolution <711> CAPSULES PF 33(4) Pg. 645 DEHYDROACETIC ACID PF 33(4) Title Pg. 703 DEHYDROACETIC ACID PF 33(4) Chemical Info Pg. 703 DEHYDROACETIC ACID PF 33(4) Definition Pg. 703 DEHYDROACETIC ACID PF 33(4) Packaging and storage Pg. 703 DEHYDROACETIC ACID PF 33(4) USP Reference standards <11> Pg. 703 DEHYDROACETIC ACID PF 33(4) Identification Pg. 703 DEHYDROACETIC ACID PF 33(4) Heavy Metals, Method II <231> Pg. 703 DEHYDROACETIC ACID PF 33(4) Loss on drying <731> Pg. 703 DEHYDROACETIC ACID PF 33(4) Melting range, Class I <741> Pg. 703 DEHYDROACETIC ACID PF 33(4) Residue on ignition <281> Pg. 703 DEHYDROACETIC ACID PF 33(4) Assay Pg. 703 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Title 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Definition 784 file:///C|/Documents%20and%20Settings/rwt/Desktop/revisions/usp31nf26secondSupplement03.html[4/26/2011 1:18:07 PM] DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Packaging and storage 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Labeling 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. USP Reference stadards 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Identification 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Uniformity of dosage units 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Loss on drying 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. -

Gene Targeting Therapies (Roy Alcalay)
Recent Developments in Gene - Targeted Therapies for Parkinson’s Disease Roy Alcalay, MD, MS Alfred and Minnie Bressler Associate Professor of Neurology Division of Movement Disorders Columbia University Medical Center Disclosures Funding: Dr. Alcalay is funded by the National Institutes of Health, the DOD, the Michael J. Fox Foundation and the Parkinson’s Foundation. Dr. Alcalay receives consultation fees from Genzyme/Sanofi, Restorbio, Janssen, and Roche. Gene Localizations Identified in PD Gene Symbol Protein Transmission Chromosome PARK1 SNCA α-synuclein AD 4q22.1 PARK2 PRKN parkin (ubiquitin ligase) AR 6q26 PARK3 ? ? AD 2p13 PARK4 SNCA triplication α-synuclein AD 4q22.1 PARK5 UCH-L1 ubiquitin C-terminal AD 4p13 hydrolase-L1 PARK6 PINK1 PTEN-induced kinase 1 AR 1p36.12 PARK7 DJ-1 DJ-1 AR 1p36.23 PARK8 LRRK2 leucine rich repeat kinase 2 AD 12q12 PARK9 ATP13A2 lysosomal ATPase AR 1p36.13 PARK10 ? ? (Iceland) AR 1p32 PARK11 GIGYF2 GRB10-interacting GYF protein 2 AD 2q37.1 PARK12 ? ? X-R Xq21-q25 PARK13 HTRA2 serine protease AD 2p13.1 PARK14 PLA2G6 phospholipase A2 (INAD) AR 22q13.1 PARK15 FBXO7 F-box only protein 7 AR 22q12.3 PARK16 ? Discovered by GWAS ? 1q32 PARK17 VPS35 vacuolar protein sorting 35 AD 16q11.2 PARK18 EIF4G1 initiation of protein synth AD 3q27.1 PARK19 DNAJC6 auxilin AR 1p31.3 PARK20 SYNJ1 synaptojanin 1 AR 21q22.11 PARK21 DNAJC13 8/RME-8 AD 3q22.1 PARK22 CHCHD2 AD 7p11.2 PARK23 VPS13C AR 15q22 Gene Localizations Identified in PD Disorder Symbol Protein Transmission Chromosome PD GBA β-glucocerebrosidase AD 1q21 SCA2 -

Genetic Control of Apigenin Di-C-Glycoside Biosynthesis in Bread Wheat Grain and Their Role
Genetic control of Apigenin di-C-glycoside biosynthesis in bread wheat grain and their role as yellow pigments of Asian alkaline noodles Submitted by Grace Yasmein Wijaya This thesis is submitted to Faculty of Sciences in fulfilment of the requirements for the degree Doctor of Philosophy Faculty of Science The University of Adelaide November 2012 This book is An Answer to Prayers of a Long list of Believers I have been very blessed and loved with all of your spiritual supports, for His guidance and protections, and sincerely will not have enough to thank you all..... May you all be blessed and loved, too As I have been always, yasmein Table of Content Table of Content i List of Tables x List of Figures xiii List of Supplemental Materials xxv Summary xxx Statement of Authorship xxxiv List of Publications xxxv Acknowledgement xxxvi List of Abbreviations xxxix Chapter I: General Introduction 1 1.1 Background 1 1.2 Knowledge Gap 2 1.3 Structure of thesis 2 Chapter II: Literature review 4 2.1 Asian noodles as one of the major end-products of Australian bread wheat 4 2.2 Yellow colour of YAN 5 2.2.1 Natural Compounds that contribute to the yellow colour of YAN 5 2.2.1.1 Types of natural compounds that contributes to the yellow colour of YAN and their roles in plants and human health 5 2.2.1.2 Contribution of xanthophylls and ACGs to the yellow colour of alkaline noodles 8 2.2.2 Factors influencing the measurement of the yellowness of noodles and the content of xanthophyll and ACG in wheat grain 10 i 2.2.3 The amount, tissue location and composition -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN. -
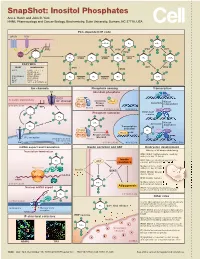
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Yeast Genome Gazetteer P35-65
gazetteer Metabolism 35 tRNA modification mitochondrial transport amino-acid metabolism other tRNA-transcription activities vesicular transport (Golgi network, etc.) nitrogen and sulphur metabolism mRNA synthesis peroxisomal transport nucleotide metabolism mRNA processing (splicing) vacuolar transport phosphate metabolism mRNA processing (5’-end, 3’-end processing extracellular transport carbohydrate metabolism and mRNA degradation) cellular import lipid, fatty-acid and sterol metabolism other mRNA-transcription activities other intracellular-transport activities biosynthesis of vitamins, cofactors and RNA transport prosthetic groups other transcription activities Cellular organization and biogenesis 54 ionic homeostasis organization and biogenesis of cell wall and Protein synthesis 48 plasma membrane Energy 40 ribosomal proteins organization and biogenesis of glycolysis translation (initiation,elongation and cytoskeleton gluconeogenesis termination) organization and biogenesis of endoplasmic pentose-phosphate pathway translational control reticulum and Golgi tricarboxylic-acid pathway tRNA synthetases organization and biogenesis of chromosome respiration other protein-synthesis activities structure fermentation mitochondrial organization and biogenesis metabolism of energy reserves (glycogen Protein destination 49 peroxisomal organization and biogenesis and trehalose) protein folding and stabilization endosomal organization and biogenesis other energy-generation activities protein targeting, sorting and translocation vacuolar and lysosomal -

Inositol (Powder) Introduced 2008
Product Information Sheet – March 2016 Inositol (powder) Introduced 2008 What Is It? Inositol (powder) Inositol is a component of the B-complex family. It supports healthy two scoops (approximately 4.2 g) contain v central nervous system function, including emotional wellness, healthy mood and behavior. Additionally, it may promote ovarian health.* inositol (as myo-inositol) .................................................................................4.2 g serving size: approximately 4.2 g (2 scoops) Uses For Inositol (powder) servings per container: 60 Emotional Wellness: Myo-inositol is the primary form of inositol 2 scoops, 1–2 times daily, with or between meals, or as directed found in the central nervous system. It plays an important role in cell by a health professional. membrane formation and serves as part of the phosphatidylinositol second messenger system, supporting serotonin, norepinenephrine and cholinergic receptor function. As a result, inositol may support healthy mood, emotional wellness and behavior, and ease occasional nervous tension.* Ovarian Function: Research suggests that myo-inositol may help to support healthy ovulatory activity, ovarian function and reproductive system function.* What Is The Source? Myo-inositol is derived from rice bran. Recommendations Pure Encapsulations® recommends 2 scoops, 1–2 times daily, with or between meals, or as directed by a health professional. Are There Any Potential Side Effects Or Precautions? Rarely, inositol has been associated with nausea, fatigue, headache or dizziness. If pregnant or lactating, consult your physician before taking this product. Are There Any Potential Drug Interactions? At this time, there are no known adverse reactions when taken in conjunction with medications. Consult your physician for more information. *These statements have not been evaluated by the Food and Drug Administration. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig.