IP3 Receptors – Lessons from Analyses Ex Cellula Ana M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autoantibodies in Neurological Diseases
Autoantibodies in neurological diseases Hu, Ri, Yo, Tr CV2 Amphiphysin Amphiphysin GM1 Ma/Ta CV2 Amphiphysin Cerebellum Intestine Hippocampus Control transfection CV2 GM2 SOX1 PNMAP 2 (MMa2/Ta) Zic4 PNMP A2 GM3G ITPR1 (MMa2/Ta) RiR CARP YoY RiR GD1G a GAD Hippocampus HEp-2 cells Cerebellum NMDAR (transf. cells) Recoverin HuH Titin Anti-Hu positive Anti-NMDA-receptor positive YoY GD1G b Recoverin Gangliosides MAG HuH GT1b SOS X1 Myelin Aquaporin-4 Titin GQ1G b MOG Zic4 VGKC (LGI1 + CASPR2) Cerebellum Intestine Cerebellum Control transfection NMDA receptors GAD65G AMPA receptors Tr (DNER) GABAB receptors DPPX Control CoC ntrol CoC ntrol IgLON5 Hippocampus HEp-2 cells Optic nerve AQP-4 (transf. cells) Glycine receptors Anti-Yo positive Anti-aquaporin-4 positive AChR Indirect immunofl uorescence EUROLINE Examples of relevant target antigens EUROIMMUN AG · Seekamp 31 · 23560 Lübeck (Germany) · Tel +49 451/5855-0 · Fax 5855-591 · [email protected] · www.euroimmun.com 2 Autoantibodies IIFT pattern Test systems Anti-Hu (ANNA-1*) IIFT: Granular fl uorescence of almost all neuronal nuclei on the substrates cerebellum and hippocampus. The Autoantibodies against basic, RNA- cell nuclei of the plexus myentericus (intestinal tissue) binding proteins of the neuronal cell are also positive. nuclei of the central and peripheral nervous system EUROLINE: Positive reaction of the recombinant Hu antigen (HuD). Associated diseases: encephalomyelitis, subacute sensory neuronopathy (Denny-Brown syndrome), autonomous neuropathy Associated tumours: small-cell lung carcinoma, Cerebellum Intestine neuroblastoma Anti-Ri (ANNA-2*) IIFT: Granular fl uorescence of almost all neuronal nuclei on the substrates cerebellum and hippocampus. The Autoantibodies against neuronal cell substrate intestine (plexus myentericus) shows no reac- nuclei of the central nervous system tion. -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

(4,5) Bisphosphate-Phospholipase C Resynthesis Cycle: Pitps Bridge the ER-PM GAP
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Topological organisation of the phosphatidylinositol (4,5) bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM GAP Shamshad Cockcroft and Padinjat Raghu* Dept. of Neuroscience, Physiology and Pharmacology, Division of Biosciences, University College London, London WC1E 6JJ, UK; *National Centre for Biological Sciences, TIFR-GKVK Campus, Bellary Road, Bangalore 560065, India Address correspondence to: Shamshad Cockcroft, University College London UK; Phone: 0044-20-7679-6259; Email: [email protected] Abstract Phospholipase C (PLC) is a receptor-regulated enzyme that hydrolyses phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) at the plasma membrane (PM) triggering three biochemical consequences, the generation of soluble inositol 1,4,5-trisphosphate (IP3), membrane– associated diacylglycerol (DG) and the consumption of plasma membrane PI(4,5)P2. Each of these three signals triggers multiple molecular processes impacting key cellular properties. The activation of PLC also triggers a sequence of biochemical reactions, collectively referred to as the PI(4,5)P2 cycle that culminates in the resynthesis of this lipid. The biochemical intermediates of this cycle and the enzymes that mediate these reactions are topologically distributed across two membrane compartments, the PM and the endoplasmic reticulum (ER). At the plasma membrane, the DG formed during PLC activation is rapidly converted to phosphatidic acid (PA) that needs to be transported to the ER where the machinery for its conversion into PI is localised. Conversely, PI from the ER needs to be rapidly transferred to the plasma membrane where it can be phosphorylated by lipid kinases to regenerate PI(4,5)P2. -

Non-Canonical Regulation of Phosphatidylserine Metabolism by a Phosphatidylinositol Transfer Protein and a Phosphatidylinositol 4-OH Kinase
bioRxiv preprint doi: https://doi.org/10.1101/696336; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Non-Canonical Regulation of Phosphatidylserine Metabolism by a Phosphatidylinositol Transfer Protein and a Phosphatidylinositol 4-OH Kinase Yaxi Wang1,2, Peihua Yuan2, Ashutosh Tripathi2, Martin Rodriguez1, Max Lönnfors2, Michal Eisenberg-Bord3, Maya Schuldiner3, and Vytas A. Bankaitis1,2,4† 1Department of Biochemistry and Biophysics Texas A&M University College Station, Texas 77843-2128 USA 2Department of Molecular and Cellular Medicine Texas A&M Health Science Center College Station, Texas 77843-1114 USA 3Department of Molecular Genetics Weizmann Institute of Science, Rehovot 7610001, Israel 4Department of Chemistry Texas A&M University College Station, Texas 77840 USA Key Words: phosphoinositides/ PITPs/ lipid kinases/ lipi metabolism/ membrane contact site † -- Corresponding author TEL: 979-436-0757 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/696336; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. ABSTRACT The phosphatidylserine (PtdSer) decarboxylase Psd2 is proposed to engage in an endoplasmic reticulum (ER)-Golgi/endosome membrane contact site (MCS) that facilitates phosphatidylserine decarboxylation to phosphatidylethanomaine (PtdEtn) in Saccharomyces cerevisiae. -

Cytosolic Phosphorylation of Calnexin Controls Intracellular Ca2ϩ Oscillations Via an Interaction with Serca2b H
Cytosolic Phosphorylation of Calnexin Controls Intracellular Ca2ϩ Oscillations via an Interaction with SERCA2b H. Llewelyn Roderick,* James D. Lechleiter,‡ and Patricia Camacho* *Department of Physiology, and ‡Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, San Antonio, Texas 78229-3900 Abstract. Calreticulin (CRT) and calnexin (CLNX) are of this residue. We also demonstrate by coimmunopre- lectin chaperones that participate in protein folding in cipitation that CLNX physically interacts with the the endoplasmic reticulum (ER). CRT is a soluble ER COOH terminus of SERCA2b and that after dephos- lumenal protein, whereas CLNX is a transmembrane phorylation treatment, this interaction is significantly protein with a cytosolic domain that contains two con- reduced. Together, our results suggest that CRT is sensus motifs for protein kinase (PK) C/proline- uniquely regulated by ER lumenal conditions, whereas directed kinase (PDK) phosphorylation. Using confo- CLNX is, in addition, regulated by the phosphorylation cal Ca2ϩ imaging in Xenopus oocytes, we report here status of its cytosolic domain. The S562 residue in that coexpression of CLNX with sarco endoplasmic CLNX acts as a molecular switch that regulates the reticulum calcium ATPase (SERCA) 2b results in inhi- interaction of the chaperone with SERCA2b, thereby bition of intracellular Ca2ϩ oscillations, suggesting a affecting Ca2ϩ signaling and controlling Ca2ϩ-sensitive functional inhibition of the pump. By site-directed mu- chaperone -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Association of the IP3R to STIM1 Provides a Reduced Intraluminal
www.nature.com/scientificreports OPEN Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, Received: 15 March 2018 Accepted: 15 May 2018 resulting in enhanced store- Published: xx xx xxxx operated calcium entry Alicia Sampieri1, Karla Santoyo1, Alexander Asanov2 & Luis Vaca 1 The involvement of inositol trisphosphate receptor (IP3R) in modulating store-operated calcium entry (SOCE) was established many years ago. Nevertheless, the molecular mechanism responsible for this observation has not been elucidated to this date. In the present study we show that IP3R associates to STIM1 upon depletion of the endoplasmic reticulum (ER) by activation of the inositol trisphosphate signaling cascade via G-protein coupled receptors. IP3R-STIM1 association results in enhanced STIM1 puncta formation and larger Orai-mediated whole-cell currents as well as increased calcium infux. Depleting the ER with a calcium ATPase inhibitor (thapsigargin, TG) does not induce IP3R-STIM1 association, indicating that this association requires an active IP3R. The IP3R-STIM1 association is only observed after IP3R activation, as evidenced by FRET experiments and co-immunoprecipitation assays. ER intraluminal calcium measurements using Mag-Fluo-4 showed enhanced calcium depletion when IP3R is overexpressed. A STIM1-GCaMP fusion protein indicates that STIM1 detects lower calcium concentrations near its EF-hand domain when IP3R is overexpressed when compared with the fuorescence reported by a GCaMP homogenously distributed in the ER lumen (ER-GCaMP). All these data together strongly suggest that activation of inositol trisphosphate signaling cascade induces the formation of the IP3R-STIM1 complex. The activated IP3R provides a reduced intraluminal calcium microenvironment near STIM1, resulting in enhanced activation of Orai currents and SOCE. -
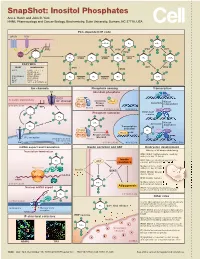
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Transcriptomic Analysis of Native Versus Cultured Human and Mouse Dorsal Root Ganglia Focused on Pharmacological Targets Short
bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia focused on pharmacological targets Short title: Comparative transcriptomics of acutely dissected versus cultured DRGs Andi Wangzhou1, Lisa A. McIlvried2, Candler Paige1, Paulino Barragan-Iglesias1, Carolyn A. Guzman1, Gregory Dussor1, Pradipta R. Ray1,#, Robert W. Gereau IV2, # and Theodore J. Price1, # 1The University of Texas at Dallas, School of Behavioral and Brain Sciences and Center for Advanced Pain Studies, 800 W Campbell Rd. Richardson, TX, 75080, USA 2Washington University Pain Center and Department of Anesthesiology, Washington University School of Medicine # corresponding authors [email protected], [email protected] and [email protected] Funding: NIH grants T32DA007261 (LM); NS065926 and NS102161 (TJP); NS106953 and NS042595 (RWG). The authors declare no conflicts of interest Author Contributions Conceived of the Project: PRR, RWG IV and TJP Performed Experiments: AW, LAM, CP, PB-I Supervised Experiments: GD, RWG IV, TJP Analyzed Data: AW, LAM, CP, CAG, PRR Supervised Bioinformatics Analysis: PRR Drew Figures: AW, PRR Wrote and Edited Manuscript: AW, LAM, CP, GD, PRR, RWG IV, TJP All authors approved the final version of the manuscript. 1 bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

(4,5)-Bisphosphate Destabilizes the Membrane of Giant Unilamellar Vesicles
5112 Biophysical Journal Volume 96 June 2009 5112–5121 Profilin Interaction with Phosphatidylinositol (4,5)-Bisphosphate Destabilizes the Membrane of Giant Unilamellar Vesicles Kannan Krishnan,† Oliver Holub,‡ Enrico Gratton,‡ Andrew H. A. Clayton,§ Stephen Cody,§ and Pierre D. J. Moens†* †Centre for Bioactive Discovery in Health and Ageing, School of Science and Technology, University of New England, Armidale, Australia; ‡Laboratory for Fluorescence Dynamics, Department of Biomedical Engineering, University of California, Irvine, California; and §Ludwig Institute for Cancer Research, Royal Melbourne Hospital, Victoria, Australia ABSTRACT Profilin, a small cytoskeletal protein, and phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] have been implicated in cellular events that alter the cell morphology, such as endocytosis, cell motility, and formation of the cleavage furrow during cytokinesis. Profilin has been shown to interact with PI(4,5)P2, but the role of this interaction is still poorly understood. Using giant unilamellar vesicles (GUVs) as a simple model of the cell membrane, we investigated the interaction between profilin and PI(4,5)P2. A number and brightness analysis demonstrated that in the absence of profilin, molar ratios of PI(4,5)P2 above 4% result in lipid demixing and cluster formations. Furthermore, adding profilin to GUVs made with 1% PI(4,5)P2 leads to the forma- tion of clusters of both profilin and PI(4,5)P2. However, due to the self-quenching of the dipyrrometheneboron difluoride-labeled PI(4,5)P2, we were unable to determine the size of these clusters. Finally, we show that the formation of these clusters results in the destabilization and deformation of the GUV membrane. -

Na+ Influx Via Orai1 Inhibits Intracellular ATP-Induced Mtorc2 Signaling to Disrupt CD4 T Cell Gene Expression and Differentiation." Elife.6
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2017 Na+ influx via Orai1 inhibits intracellular ATP- induced mTORC2 signaling to disrupt CD4 T cell gene expression and differentiation Yong Miao Washington University School of Medicine in St. Louis Jaya Bhushan Washington University School of Medicine in St. Louis Adish Dani Washington University School of Medicine in St. Louis Monika Vig Washington University School of Medicine in St. Louis Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Miao, Yong; Bhushan, Jaya; Dani, Adish; and Vig, Monika, ,"Na+ influx via Orai1 inhibits intracellular ATP-induced mTORC2 signaling to disrupt CD4 T cell gene expression and differentiation." Elife.6,. e25155. (2017). https://digitalcommons.wustl.edu/open_access_pubs/6064 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. RESEARCH ARTICLE Na+ influx via Orai1 inhibits intracellular ATP-induced mTORC2 signaling to disrupt CD4 T cell gene expression and differentiation Yong Miao1, Jaya Bhushan1, Adish Dani1,2, Monika Vig1* 1Department of Pathology and Immunology, Washington University School of Medicine, St Louis, United States; 2Hope Center for Neurological Disorders, Washington University School of Medicine, St Louis, United States Abstract T cell effector functions require sustained calcium influx. However, the signaling and phenotypic consequences of non-specific sodium permeation via calcium channels remain unknown. a-SNAP is a crucial component of Orai1 channels, and its depletion disrupts the functional assembly of Orai1 multimers. -

Transcriptome Sequencing and Genome-Wide Association Analyses Reveal Lysosomal Function and Actin Cytoskeleton Remodeling in Schizophrenia and Bipolar Disorder
Molecular Psychiatry (2015) 20, 563–572 © 2015 Macmillan Publishers Limited All rights reserved 1359-4184/15 www.nature.com/mp ORIGINAL ARTICLE Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder Z Zhao1,6,JXu2,6, J Chen3,6, S Kim4, M Reimers3, S-A Bacanu3,HYu1, C Liu5, J Sun1, Q Wang1, P Jia1,FXu2, Y Zhang2, KS Kendler3, Z Peng2 and X Chen3 Schizophrenia (SCZ) and bipolar disorder (BPD) are severe mental disorders with high heritability. Clinicians have long noticed the similarities of clinic symptoms between these disorders. In recent years, accumulating evidence indicates some shared genetic liabilities. However, what is shared remains elusive. In this study, we conducted whole transcriptome analysis of post-mortem brain tissues (cingulate cortex) from SCZ, BPD and control subjects, and identified differentially expressed genes in these disorders. We found 105 and 153 genes differentially expressed in SCZ and BPD, respectively. By comparing the t-test scores, we found that many of the genes differentially expressed in SCZ and BPD are concordant in their expression level (q ⩽ 0.01, 53 genes; q ⩽ 0.05, 213 genes; q ⩽ 0.1, 885 genes). Using genome-wide association data from the Psychiatric Genomics Consortium, we found that these differentially and concordantly expressed genes were enriched in association signals for both SCZ (Po10 − 7) and BPD (P = 0.029). To our knowledge, this is the first time that a substantially large number of genes show concordant expression and association for both SCZ and BPD. Pathway analyses of these genes indicated that they are involved in the lysosome, Fc gamma receptor-mediated phagocytosis, regulation of actin cytoskeleton pathways, along with several cancer pathways.