Procedure Codes, Section 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
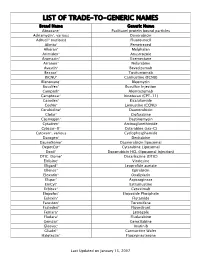
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Novel Formulations for Treatment of Migraine
(19) TZZ _T (11) EP 2 756 756 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.07.2014 Bulletin 2014/30 A01N 43/42 (2006.01) A61K 31/44 (2006.01) (21) Application number: 14165112.5 (22) Date of filing: 24.04.2009 (84) Designated Contracting States: • Turanin, John AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Emeryville, CA California 94608 (US) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Hawley, Roger PT RO SE SI SK TR Emeryville, CA California 94608 (US) • Schuster, Jeffrey, A. (30) Priority: 28.04.2008 US 48463 Bolinas, CA California 94924 (US) (62) Document number(s) of the earlier application(s) in (74) Representative: Duxbury, Stephen et al accordance with Art. 76 EPC: Arnold & Siedsma 09739139.5 / 2 273 880 Pettenkoferstrasse 37 80336 München (DE) (71) Applicant: Zogenix, Inc. Emeryville CA 94608 (US) Remarks: This application was filed on 17-04-2014 as a (72) Inventors: divisional application to the application mentioned • Farr, Stephen J. under INID code 62. Orinda, CA California 94563 (US) (54) Novel formulations for treatment of migraine (57) Systems and methods are described for treating Systems that are self contained, portable, prefilled, and un-met medical needs in migraine and related conditions simple to self administer at the onset of a migraine attack such as cluster headache. Included are treatments that are disclosed, and preferably include a needle-free in- are both rapid onset and long acting, which include sus- jector and a high viscosity formulation, to eliminate such tained release formulations, and combination products, issues as fear of self administration with needles, and Also included are treatments for multiple symptoms of needle stick and cross contamination. -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-Induced Nausea and Vomiting in Children with Cancer
This is a repository copy of Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-induced Nausea and Vomiting in Children with Cancer. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/97043/ Version: Accepted Version Article: Flank, Jaqueline, Robinson, Paula, Holdsworth, Mark et al. (7 more authors) (2016) Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-induced Nausea and Vomiting in Children with Cancer. Pediatric blood & cancer. ISSN 1545-5009 https://doi.org/10.1002/pbc.25955 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Pediatric Blood & Cancer Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-induced Nausea and Vomiting in Children with Cancer Journal: Pediatric Blood & Cancer Manuscript ID PBC-15-1233.R1 Wiley - ManuscriptFor -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Parkinson's Disease Fact Sheet
Parkinson’s Disease Fact Sheet About Parkinson’s Disease Parkinson’s disease is a progressive, incurable neurological disorder associated with a loss of dopamine-generating cells in the brain. It is primarily associated with progressive loss of motor control, but it results in a complex array of symptoms, including many non-motor symptoms. Parkinson’s impacts an estimated one million people in the United States. Critical Clinical Care Considerations • To avoid serious side effects, Parkinson’s patients need their medications on time, every time — do not skip or postpone doses. • Write down the exact times of day medications are to be administered so that doses are given on the same schedule the patient follows at home. • Do not substitute Parkinson’s medications or stop levodopa therapy abruptly. • Resume medications immediately following procedures, unless vomiting or severely incapacitated. • If an antipsychotic is necessary, use pimavanserin (Nuplazid), quetiapine (Seroquel) or clozapine (Clozaril). • Be alert for symptoms of dysphagia (trouble swallowing) and risk of pneumonia. • Ambulate as soon as medically safe. Patients may require assistance. Common Symptoms of Parkinson’s Disease Motor Non-Motor • Shaking or tremor at rest • Depression • Bradykinesia or freezing (being stuck • Anxiety in place when attempting to walk) • Constipation • Low voice volume or muffled speech • Cognitive decline and dementia • Lack of facial expression • Impulse control disorders • Stiffness or rigidity of the arms, legs • Orthostatic hypotension or -

Thames Valley Chemotherapy Regimens Sarcoma
Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Chemotherapy Regimens– Sarcoma 1 of 98 Thames Valley Notes from the editor All chemotherapy regimens, and associated guidelines eg antiemetics and dose bands are available on the Network website www.tvscn.nhs.uk/networks/cancer-topics/chemotherapy/ Any correspondence about the regimens should be addressed to: Sally Coutts, Cancer Pharmacist, Thames Valley email: [email protected] Acknowledgements These regimens have been compiled by the Network Pharmacy Group in collaboration with key contribution from Prof Bass Hassan, Medical Oncologist, OUH Dr Sally Trent, Clinical Oncologist, OUH Dr James Gildersleve, Clinical Oncologist, RBFT Dr Sarah Pratap, Medical Oncologist, OUH Dr Shaun Wilson, TYA - Paediatric Oncologist, OUH Catherine Chaytor, Cancer Pharmacist, OUH Varsha Ormerod, Cancer Pharmacist, OUH Kristen Moorhouse, Cancer Pharmacist, OUH © Thames Valley Cancer Network. All rights reserved. Not to be reproduced in whole or in part without the permission of the copyright owner. Chemotherapy Regimens– Sarcoma 2 of 98 Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Network Chemotherapy Regimens used in the management of Sarcoma Date published: January 2019 Date of review: June 2022 Chemotherapy Regimens Name of regimen Indication Page List of amendments to this version 5 Imatinib GIST 6 Sunitinib GIST 9 Regorafenib GIST 11 Paclitaxel weekly (Taxol) Angiosarcoma 13 AC Osteosarcoma 15 Cisplatin Imatinib – if local Trust funding agreed Chordoma 18 Doxorubicin Sarcoma 21 -

(12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya Et Al
US 2006.0024.365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya et al. (43) Pub. Date: Feb. 2, 2006 (54) NOVEL DOSAGE FORM (30) Foreign Application Priority Data (76) Inventors: Navin Vaya, Gujarat (IN); Rajesh Aug. 5, 2002 (IN)................................. 699/MUM/2002 Singh Karan, Gujarat (IN); Sunil Aug. 5, 2002 (IN). ... 697/MUM/2002 Sadanand, Gujarat (IN); Vinod Kumar Jan. 22, 2003 (IN)................................... 80/MUM/2003 Gupta, Gujarat (IN) Jan. 22, 2003 (IN)................................... 82/MUM/2003 Correspondence Address: Publication Classification HEDMAN & COSTIGAN P.C. (51) Int. Cl. 1185 AVENUE OF THE AMERICAS A6IK 9/22 (2006.01) NEW YORK, NY 10036 (US) (52) U.S. Cl. .............................................................. 424/468 (22) Filed: May 19, 2005 A dosage form comprising of a high dose, high Solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of Related U.S. Application Data immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of (63) Continuation-in-part of application No. 10/630,446, modified release active ingredient per unit is from 500 mg to filed on Jul. 29, 2003. 1500 mg, a process for preparing the dosage form. Patent Application Publication Feb. 2, 2006 Sheet 1 of 10 US 2006/0024.365A1 FIGURE 1 FIGURE 2 FIGURE 3 Patent Application Publication Feb. 2, 2006 Sheet 2 of 10 US 2006/0024.365A1 FIGURE 4 (a) 7 FIGURE 4 (b) Patent Application Publication Feb. 2, 2006 Sheet 3 of 10 US 2006/0024.365 A1 FIGURE 5 100 ov -- 60 40 20 C 2 4. -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

Cardiotoxicity of Doxorubicin Is Mediated Through Mitochondrial Iron Accumulation
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa, … , Tejaswitha Jairaj Naik, Hossein Ardehali J Clin Invest. 2014;124(2):617-630. https://doi.org/10.1172/JCI72931. Research Article Cardiology Doxorubicin is an effective anticancer drug with known cardiotoxic side effects. It has been hypothesized that doxorubicin- dependent cardiotoxicity occurs through ROS production and possibly cellular iron accumulation. Here, we found that cardiotoxicity develops through the preferential accumulation of iron inside the mitochondria following doxorubicin treatment. In isolated cardiomyocytes, doxorubicin became concentrated in the mitochondria and increased both mitochondrial iron and cellular ROS levels. Overexpression of ABCB8, a mitochondrial protein that facilitates iron export, in vitro and in the hearts of transgenic mice decreased mitochondrial iron and cellular ROS and protected against doxorubicin-induced cardiomyopathy. Dexrazoxane, a drug that attenuates doxorubicin-induced cardiotoxicity, decreased mitochondrial iron levels and reversed doxorubicin-induced cardiac damage. Finally, hearts from patients with doxorubicin-induced cardiomyopathy had markedly higher mitochondrial iron levels than hearts from patients with other types of cardiomyopathies or normal cardiac function. These results suggest that the cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin- induced cardiomyopathy. Find the latest version: https://jci.me/72931/pdf Research article Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa,1 Mohsen Ghanefar,1 Marina Bayeva,1 Rongxue Wu,1 Arineh Khechaduri,1 Sathyamangla V. Naga Prasad,2 R. Kannan Mutharasan,1 Tejaswitha Jairaj Naik,1 and Hossein Ardehali1 1Feinberg Cardiovascular Institute, Northwestern University School of Medicine, Chicago, Illinois, USA.