Thames Valley Chemotherapy Regimens Sarcoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASCC/ESMO ANTIEMETIC GUIDELINE 2016 with Updates in 2019
1 ANTIEMETIC GUIDELINES: MASCC/ESMO MASCC/ESMO ANTIEMETIC GUIDELINE 2016 With Updates in 2019 Organizing and Overall Meeting Chairs: Matti Aapro, MD Richard J. Gralla, MD Jørn Herrstedt, MD, DMSci Alex Molassiotis, RN, PhD Fausto Roila, MD © Multinational Association of Supportive Care in CancerTM All rights reserved worldwide. 2 ANTIEMETIC GUIDELINES: MASCC/ESMO These slides are provided to all by the Multinational Association of Supportive Care in Cancer and can be used freely, provided no changes are made and the MASCC and ESMO logos, as well as date of the information are retained. For questions please contact: Matti Aapro at [email protected] Chair, MASCC Antiemetic Study Group or Alex Molassiotis at [email protected] Past Chair, MASCC Antiemetic Study Group 3 ANTIEMETIC GUIDELINES: MASCC/ESMO Consensus A few comments on this guideline set: • This set of guideline slides represents the latest edition of the guideline process. • This set of slides has been endorsed by the MASCC Antiemetic Guideline Committee and ESMO Guideline Committee. • The guidelines are based on the votes of the panel at the Copenhagen Consensus Conference on Antiemetic Therapy, June 2015. • Latest version: March 2016, with updates in 2019. 4 ANTIEMETIC GUIDELINES: MASCC/ESMO Changes: The Steering Committee has clarified some points: 2016: • A footnote clarified that aprepitant 165 mg is approved by regulatory authorities in some parts of the world ( although no randomised clinical trial has investigated this dose ). Thus use of aprepitant 80 mg in the delayed phase is only for those cases where aprepitant 125 mg is used on day 1. • A probable modification in pediatric guidelines based on the recent Cochrane meta-analysis is indicated. -

Specific Effects of Trabectedin and Lurbinectedin on Human Macrophage Function and Fate—Novel Insights
cancers Article Specific Effects of Trabectedin and Lurbinectedin on Human Macrophage Function and Fate—Novel Insights 1, 1, 1 Adrián Povo-Retana y, Marina Mojena y, Adrian B. Stremtan , Victoria B. Fernández-García 1, Ana Gómez-Sáez 1, Cristina Nuevo-Tapioles 2,3 , José M. Molina-Guijarro 4 , José Avendaño-Ortiz 5, José M. Cuezva 2,3 , Eduardo López-Collazo 5, Juan F. Martínez-Leal 4 and Lisardo Boscá 1,5,6,* 1 Instituto de Investigaciones Biomédicas Alberto Sols (Centro Mixto CSIC-UAM), 28029 Madrid, Spain; [email protected] (A.P.-R.); [email protected] (M.M.); [email protected] (A.B.S.); [email protected] (V.B.F.-G.); [email protected] (A.G.-S.) 2 Centro de Biología Molecular (Centro Mixto CSIC-UAM), Nicolás Cabrera S/N, Ciudad Universitaria de Cantoblanco, 28049 Madrid, Spain; [email protected] (C.N.-T.); [email protected] (J.M.C.) 3 Centro de Investigación Biomédica en Red en Enfermedades Raras (CIBERER), 28029 Madrid, Spain 4 Pharma Mar SA, 28770 Colmenar Viejo, Spain; [email protected] (J.M.M.-G.); [email protected] (J.F.M.-L.) 5 Instituto de Investigación Sanitaria La Paz (IdiPaz), Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] (J.A.-O.); [email protected] (E.L.-C.) 6 Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares (CIBERCV), 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-9149-72747 These authors have equal contribution. y Received: 28 August 2020; Accepted: 16 October 2020; Published: 20 October 2020 Simple Summary: Trabectedin and lurbinectedin are two potent onco-therapeutic drugs for the treatment of advanced soft tissue sarcomas. -

To Induce Cytotoxicity of Ovarian Cancer Cells Through Increased Autophagy and Apoptosis
Endocrine-Related Cancer (2012) 19 711–723 Arsenic trioxide synergizes with everolimus (Rad001) to induce cytotoxicity of ovarian cancer cells through increased autophagy and apoptosis Nan Liu1,2, Sheng Tai2,4, Boxiao Ding2, Ryan K Thor2, Sunita Bhuta2, Yin Sun2 and Jiaoti Huang2,3 1Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, Guangdong 510515, People’s Republic of China 2Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at the University of California at Los Angeles, 10833 Le Conte Avenue, 13-229 CHS, Los Angeles, California 90095-1732, USA 3Jonsson Comprehensive Cancer Center and Broad Center for Regenerative Medicine and Stem Cell Biology, David Geffen School of Medicine at the University of California at Los Angeles, Los Angeles, California, USA 4Department of Urology and Anhui Geriatric Institute, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China (Correspondence should be addressed to N Liu at Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University; Email: [email protected]; J Huang at Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at the University of California at Los Angeles; Email: [email protected]) Abstract Phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway plays a key role in the tumorigenesis of a variety of human cancers including ovarian cancer. However, inhibitors of this pathway such as Rad001 have not shown therapeutic efficacy as a single agent for this cancer. Arsenic trioxide (ATO) induces an autophagic pathway in ovarian carcinoma cells. We found that ATO can synergize with Rad001 to induce cytotoxicity of ovarian cancer cells. -

Topotecan, Pegylated Liposomal Doxorubicin Hydrochloride, Paclitaxel, Trabectedin and Gemcitabine for Treating Recurrent Ovarian Cancer
Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer Information for the public Published: TBC nice.org.uk What has NICE said? For recurrent ovarian cancer, the following possible treatments are recommended: paclitaxel (also known as Taxol) on its own or with platinum pegylated liposomal doxorubicin hydrochloride (PLDH, also known as Caelyx) on its own or with platinum. For ovarian cancer that has recurred for the first time and is platinum-sensitive, Nice does not recommend gemcitabine (Gemzar) with carboplatin (Paraplatin), trabectedin (Yondelis) with PLDH, or topotecan (Hycamtin or Potactasol). Topotecan is also not recommended for treating recurrent ovarian cancer that is platinum-resistant or platinum-refractory. What does this mean for me? If you have recurrent ovarian cancer and your doctor thinks that paclitaxel on its own or with platinum, or pegylated liposomal doxorubicin hydrochloride (PLDH) on its own, is the right treatment, you should be able to have the treatment on the NHS. These treatments should be available on the NHS within 3 months of the guidance being issued. © NICE TBC. All rights reserved. Page 1 of 3 Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer You may be able to have PLDH with platinum treatment on the NHS as long as your doctor gets your written consent to have it and the NHS within your area agrees to provide it. If you are already taking gemcitabine with carboplatin, trabectedin with PLDH, or topotecan for recurrent ovarian cancer, you should be able to continue taking it until you and your doctor decide it is the right time to stop. -

Pegylated Liposomal Doxorubicin Hydrochloride, Paclitaxel, Trabectedin and Gemcitabine for Treating Recurrent Ovarian Cancer
Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer Technology appraisal guidance Published: 26 April 2016 nice.org.uk/guidance/ta389 © NICE 2016. All rights reserved. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer (TA389) Contents 1 Recommendations ......................................................................................................................................................... 3 2 The technologies............................................................................................................................................................. 5 Gemcitabine....................................................................................................................................................................................... 5 Paclitaxel ............................................................................................................................................................................................. 5 Pegylated liposomal doxorubicin hydrochloride................................................................................................................. 6 Topotecan............................................................................................................................................................................................ 7 Trabectedin........................................................................................................................................................................................ -

YONDELIS (Trabectedin) for Injection, for Intravenous Use Embryofetal Toxicity: Can Cause Fetal Harm
Hepatotoxicity: Hepatotoxicity may occur. Monitor and delay and/or HIGHLIGHTS OF PRESCRIBING INFORMATION reduce dose if needed (5.3) These highlights do not include all the information needed to use Cardiomyopathy: Severe and fatal cardiomyopathy can occur. Withhold YONDELIS® safely and effectively. See full prescribing information for YONDELIS in patients with left ventricular dysfunction (5.4) YONDELIS. Capillary leak syndrome: Monitor and discontinue YONDELIS for capillary leak syndrome (5.5) YONDELIS (trabectedin) for injection, for intravenous use Embryofetal toxicity: Can cause fetal harm. Advise of potential risk to a Initial U.S. Approval: 2015 fetus and use effective contraception (5.7, 8.1, 8.3) ----------------------------INDICATIONS AND USAGE---------------------------- ------------------------------ADVERSE REACTIONS------------------------------- YONDELIS is an alkylating drug indicated for the treatment of patients with The most common (≥20%) adverse reactions are nausea, fatigue, vomiting, unresectable or metastatic liposarcoma or leiomyosarcoma who received a constipation, decreased appetite, diarrhea, peripheral edema, dyspnea, and prior anthracycline-containing regimen (1) headache. The most common (5%) grades 3-4 laboratory abnormalities are: -----------------------DOSAGE AND ADMINISTRATION----------------------- neutropenia, increased ALT, thrombocytopenia, anemia, increased AST, and Administer at 1.5 mg/m2 body surface area as a 24-hour intravenous increased creatine phosphokinase. (6.1) infusion, every 3 weeks through a central venous line (2.1, 2.5) Premedication: dexamethasone 20 mg intravenously, 30 min before each To report SUSPECTED ADVERSE REACTIONS, contact Janssen infusion (2.2) Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA- Hepatic Impairment: Administer at 0.9 mg/m2 body surface area as a 1088 or www.fda.gov/medwatch. -

Cardiotoxicity of Doxorubicin Is Mediated Through Mitochondrial Iron Accumulation
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa, … , Tejaswitha Jairaj Naik, Hossein Ardehali J Clin Invest. 2014;124(2):617-630. https://doi.org/10.1172/JCI72931. Research Article Cardiology Doxorubicin is an effective anticancer drug with known cardiotoxic side effects. It has been hypothesized that doxorubicin- dependent cardiotoxicity occurs through ROS production and possibly cellular iron accumulation. Here, we found that cardiotoxicity develops through the preferential accumulation of iron inside the mitochondria following doxorubicin treatment. In isolated cardiomyocytes, doxorubicin became concentrated in the mitochondria and increased both mitochondrial iron and cellular ROS levels. Overexpression of ABCB8, a mitochondrial protein that facilitates iron export, in vitro and in the hearts of transgenic mice decreased mitochondrial iron and cellular ROS and protected against doxorubicin-induced cardiomyopathy. Dexrazoxane, a drug that attenuates doxorubicin-induced cardiotoxicity, decreased mitochondrial iron levels and reversed doxorubicin-induced cardiac damage. Finally, hearts from patients with doxorubicin-induced cardiomyopathy had markedly higher mitochondrial iron levels than hearts from patients with other types of cardiomyopathies or normal cardiac function. These results suggest that the cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin- induced cardiomyopathy. Find the latest version: https://jci.me/72931/pdf Research article Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa,1 Mohsen Ghanefar,1 Marina Bayeva,1 Rongxue Wu,1 Arineh Khechaduri,1 Sathyamangla V. Naga Prasad,2 R. Kannan Mutharasan,1 Tejaswitha Jairaj Naik,1 and Hossein Ardehali1 1Feinberg Cardiovascular Institute, Northwestern University School of Medicine, Chicago, Illinois, USA. -

ZINECARD® (Dexrazoxane) for Injection Regimens
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------DOSAGE FORMS AND STRENGTHS------------- These highlights do not include all the information needed to use 250 mg or 500 mg single dose vials as sterile, pyrogen-free lyophilizates. (3) ZINECARD safely and effectively. See full prescribing information for ZINECARD. -------------------------------CONTRAINDICATIONS------------------------------ ZINECARD should not be used with non-anthracycline chemotherapy ZINECARD® (dexrazoxane) for injection regimens. (4) Initial U.S. Approval: 1995 -----------------------WARNINGS AND PRECAUTIONS------------------------ ---------------------------INDICATIONS AND USAGE----------------------- Myelosuppression: ZINECARD may increase the myelosuppresive ZINECARD is a cytoprotective agent indicated for reducing the incidence and effects of chemotherapeutic agents. Perform hematological monitoring. severity of cardiomyopathy associated with doxorubicin administration in (5.1) women with metastatic breast cancer who have received a cumulative Embryo-Fetal Toxicity: Can cause fetal harm. Advise female patients of doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin reproductive potential of the potential hazard to the fetus. (5.5, 8.1) therapy to maintain tumor control. Do not use ZINECARD with doxorubicin initiation. (1) ------------------------------ADVERSE REACTIONS------------------------------- In clinical studies, ZINECARD was administered to patients also receiving -----------------------DOSAGE AND ADMINISTRATION---------------------- chemotherapeutic agents for cancer. Pain on injection was observed more Reconstitute vial contents and dilute before use. (2.3) frequently in patients receiving ZINECARD versus placebo. (6.1) Administer ZINECARD by intravenous infusion over 15 minutes. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH. (2.1, 2.3) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at The recommended dosage ratio of ZINECARD to doxorubicin is 10:1 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

Cancer Drugs Used Today
The American Society of Pharmacognosy Barry R. O’Keefe, Executive Committee American Society of Pharmacognosy Brandcenter, Virginia Commonwealth University, Richmond, VA, September 26, 2014 The American Society of Pharmacognosy Founded in 1959 in Chicago, IL to “promote the growth and development of pharmacognosy, to provide opportunity for association among workers in science, to provide opportunities for presentation of research achievements, and to promote the publication of meritorious research.” The American Society of Pharmacognosy The Premier Society in the United States Devoted to the Study of Natural Products • ASP members have been responsible for the discovery of several of the most important anti-cancer drugs used today. • Almost all of the currently used antibiotics have been derived from natural products. • Natural products are also the templates for antiviral, anti-cholesterol, anti-diabetic, anti-malaria and immunosuppressive agents as well as pain medications. • ASP members are also active in chemical ecology, biodiversity, responsible sourcing and sustainable development of plants, animals, microbes and marine organisms. Why Should You Care About Pharmacognosy? The World’s Forests and Oceans are Rapidly Being Depleted of Unique Species. Natural Products Research and the ASP in Particular Support Biodiversity Efforts Around the Globe. Why Should You Care About Natural Products? >50% of antimicrobial and anti-cancer drugs come from natural products. All Small Molecule Drugs Most large pharmaceutical companies have eliminated -
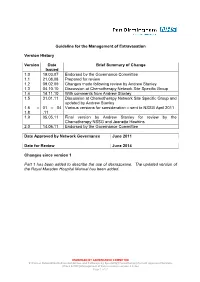
Guideline for the Management of Extravasation
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane. -

Upfront Dexrazoxane for the Reduction of Anthracycline-Induced
Ganatra et al. Cardio-Oncology (2019) 5:1 https://doi.org/10.1186/s40959-019-0036-7 RESEARCH Open Access Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series Sarju Ganatra1,2,3*, Anju Nohria3, Sachin Shah2, John D. Groarke3, Ajay Sharma2, David Venesy2, Richard Patten2, Krishna Gunturu4,5, Corrine Zarwan4, Tomas G. Neilan6, Ana Barac7, Salim S. Hayek8, Sourbha Dani9, Shantanu Solanki10, Syed Saad Mahmood11 and Steven E. Lipshultz12 Abstract Background: Cardiotoxicity associated with anthracycline-based chemotherapies has limited their use in patients with preexisting cardiomyopathy or heart failure. Dexrazoxane protects against the cardiotoxic effects of anthracyclines, but in the USA and some European countries, its use had been restricted to adults with advanced breast cancer receiving a cumulative doxorubicin (an anthracycline) dose > 300 mg/m2. We evaluated the off-label use of dexrazoxane as a cardioprotectant in adult patients with preexisting cardiomyopathy, undergoing anthracycline chemotherapy. Methods: Between July 2015 and June 2017, five consecutive patients, with preexisting, asymptomatic, systolic left ventricular (LV) dysfunction who required anthracycline-based chemotherapy, were concomitantly treated with off-label dexrazoxane, administered 30 min before each anthracycline dose, regardless of cancer type or stage. Demographic, cardiovascular, and cancer-related outcomes were compared to those of three consecutive patients with asymptomatic cardiomyopathy treated earlier at the same hospital without dexrazoxane. Results: Mean age of the five dexrazoxane-treated patients and three patients treated without dexrazoxane was 70.6 and 72.6 years, respectively. All five dexrazoxane-treated patients successfully completed their planned chemotherapy (doxorubicin, 280 to 300 mg/m2). -

Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases
cells Review Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases George J. Kontoghiorghes * and Christina N. Kontoghiorghe Postgraduate Research Institute of Science, Technology, Environment and Medicine, CY-3021 Limassol, Cyprus * Correspondence: [email protected]; Tel./Fax: +357-2627-2076 Received: 7 May 2020; Accepted: 5 June 2020; Published: 12 June 2020 Abstract: Iron is essential for all living organisms. Many iron-containing proteins and metabolic pathways play a key role in almost all cellular and physiological functions. The diversity of the activity and function of iron and its associated pathologies is based on bond formation with adjacent ligands and the overall structure of the iron complex in proteins or with other biomolecules. The control of the metabolic pathways of iron absorption, utilization, recycling and excretion by iron-containing proteins ensures normal biologic and physiological activity. Abnormalities in iron-containing proteins, iron metabolic pathways and also other associated processes can lead to an array of diseases. These include iron deficiency, which affects more than a quarter of the world’s population; hemoglobinopathies, which are the most common of the genetic disorders and idiopathic hemochromatosis. Iron is the most common catalyst of free radical production and oxidative stress which are implicated in tissue damage in most pathologic conditions, cancer initiation and progression, neurodegeneration and many other diseases. The interaction of iron and iron-containing proteins with dietary and xenobiotic molecules, including drugs, may affect iron metabolic and disease processes. Deferiprone, deferoxamine, deferasirox and other chelating drugs can offer therapeutic solutions for most diseases associated with iron metabolism including iron overload and deficiency, neurodegeneration and cancer, the detoxification of xenobiotic metals and most diseases associated with free radical pathology.