ZINECARD® (Dexrazoxane) for Injection Regimens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thames Valley Chemotherapy Regimens Sarcoma
Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Chemotherapy Regimens– Sarcoma 1 of 98 Thames Valley Notes from the editor All chemotherapy regimens, and associated guidelines eg antiemetics and dose bands are available on the Network website www.tvscn.nhs.uk/networks/cancer-topics/chemotherapy/ Any correspondence about the regimens should be addressed to: Sally Coutts, Cancer Pharmacist, Thames Valley email: [email protected] Acknowledgements These regimens have been compiled by the Network Pharmacy Group in collaboration with key contribution from Prof Bass Hassan, Medical Oncologist, OUH Dr Sally Trent, Clinical Oncologist, OUH Dr James Gildersleve, Clinical Oncologist, RBFT Dr Sarah Pratap, Medical Oncologist, OUH Dr Shaun Wilson, TYA - Paediatric Oncologist, OUH Catherine Chaytor, Cancer Pharmacist, OUH Varsha Ormerod, Cancer Pharmacist, OUH Kristen Moorhouse, Cancer Pharmacist, OUH © Thames Valley Cancer Network. All rights reserved. Not to be reproduced in whole or in part without the permission of the copyright owner. Chemotherapy Regimens– Sarcoma 2 of 98 Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Network Chemotherapy Regimens used in the management of Sarcoma Date published: January 2019 Date of review: June 2022 Chemotherapy Regimens Name of regimen Indication Page List of amendments to this version 5 Imatinib GIST 6 Sunitinib GIST 9 Regorafenib GIST 11 Paclitaxel weekly (Taxol) Angiosarcoma 13 AC Osteosarcoma 15 Cisplatin Imatinib – if local Trust funding agreed Chordoma 18 Doxorubicin Sarcoma 21 -

Cardiotoxicity of Doxorubicin Is Mediated Through Mitochondrial Iron Accumulation
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa, … , Tejaswitha Jairaj Naik, Hossein Ardehali J Clin Invest. 2014;124(2):617-630. https://doi.org/10.1172/JCI72931. Research Article Cardiology Doxorubicin is an effective anticancer drug with known cardiotoxic side effects. It has been hypothesized that doxorubicin- dependent cardiotoxicity occurs through ROS production and possibly cellular iron accumulation. Here, we found that cardiotoxicity develops through the preferential accumulation of iron inside the mitochondria following doxorubicin treatment. In isolated cardiomyocytes, doxorubicin became concentrated in the mitochondria and increased both mitochondrial iron and cellular ROS levels. Overexpression of ABCB8, a mitochondrial protein that facilitates iron export, in vitro and in the hearts of transgenic mice decreased mitochondrial iron and cellular ROS and protected against doxorubicin-induced cardiomyopathy. Dexrazoxane, a drug that attenuates doxorubicin-induced cardiotoxicity, decreased mitochondrial iron levels and reversed doxorubicin-induced cardiac damage. Finally, hearts from patients with doxorubicin-induced cardiomyopathy had markedly higher mitochondrial iron levels than hearts from patients with other types of cardiomyopathies or normal cardiac function. These results suggest that the cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin- induced cardiomyopathy. Find the latest version: https://jci.me/72931/pdf Research article Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa,1 Mohsen Ghanefar,1 Marina Bayeva,1 Rongxue Wu,1 Arineh Khechaduri,1 Sathyamangla V. Naga Prasad,2 R. Kannan Mutharasan,1 Tejaswitha Jairaj Naik,1 and Hossein Ardehali1 1Feinberg Cardiovascular Institute, Northwestern University School of Medicine, Chicago, Illinois, USA. -
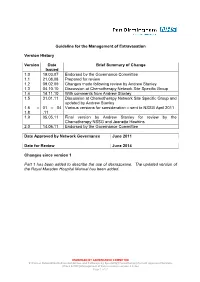
Guideline for the Management of Extravasation
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane. -

Upfront Dexrazoxane for the Reduction of Anthracycline-Induced
Ganatra et al. Cardio-Oncology (2019) 5:1 https://doi.org/10.1186/s40959-019-0036-7 RESEARCH Open Access Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series Sarju Ganatra1,2,3*, Anju Nohria3, Sachin Shah2, John D. Groarke3, Ajay Sharma2, David Venesy2, Richard Patten2, Krishna Gunturu4,5, Corrine Zarwan4, Tomas G. Neilan6, Ana Barac7, Salim S. Hayek8, Sourbha Dani9, Shantanu Solanki10, Syed Saad Mahmood11 and Steven E. Lipshultz12 Abstract Background: Cardiotoxicity associated with anthracycline-based chemotherapies has limited their use in patients with preexisting cardiomyopathy or heart failure. Dexrazoxane protects against the cardiotoxic effects of anthracyclines, but in the USA and some European countries, its use had been restricted to adults with advanced breast cancer receiving a cumulative doxorubicin (an anthracycline) dose > 300 mg/m2. We evaluated the off-label use of dexrazoxane as a cardioprotectant in adult patients with preexisting cardiomyopathy, undergoing anthracycline chemotherapy. Methods: Between July 2015 and June 2017, five consecutive patients, with preexisting, asymptomatic, systolic left ventricular (LV) dysfunction who required anthracycline-based chemotherapy, were concomitantly treated with off-label dexrazoxane, administered 30 min before each anthracycline dose, regardless of cancer type or stage. Demographic, cardiovascular, and cancer-related outcomes were compared to those of three consecutive patients with asymptomatic cardiomyopathy treated earlier at the same hospital without dexrazoxane. Results: Mean age of the five dexrazoxane-treated patients and three patients treated without dexrazoxane was 70.6 and 72.6 years, respectively. All five dexrazoxane-treated patients successfully completed their planned chemotherapy (doxorubicin, 280 to 300 mg/m2). -

Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases
cells Review Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases George J. Kontoghiorghes * and Christina N. Kontoghiorghe Postgraduate Research Institute of Science, Technology, Environment and Medicine, CY-3021 Limassol, Cyprus * Correspondence: [email protected]; Tel./Fax: +357-2627-2076 Received: 7 May 2020; Accepted: 5 June 2020; Published: 12 June 2020 Abstract: Iron is essential for all living organisms. Many iron-containing proteins and metabolic pathways play a key role in almost all cellular and physiological functions. The diversity of the activity and function of iron and its associated pathologies is based on bond formation with adjacent ligands and the overall structure of the iron complex in proteins or with other biomolecules. The control of the metabolic pathways of iron absorption, utilization, recycling and excretion by iron-containing proteins ensures normal biologic and physiological activity. Abnormalities in iron-containing proteins, iron metabolic pathways and also other associated processes can lead to an array of diseases. These include iron deficiency, which affects more than a quarter of the world’s population; hemoglobinopathies, which are the most common of the genetic disorders and idiopathic hemochromatosis. Iron is the most common catalyst of free radical production and oxidative stress which are implicated in tissue damage in most pathologic conditions, cancer initiation and progression, neurodegeneration and many other diseases. The interaction of iron and iron-containing proteins with dietary and xenobiotic molecules, including drugs, may affect iron metabolic and disease processes. Deferiprone, deferoxamine, deferasirox and other chelating drugs can offer therapeutic solutions for most diseases associated with iron metabolism including iron overload and deficiency, neurodegeneration and cancer, the detoxification of xenobiotic metals and most diseases associated with free radical pathology. -

Anthracycline Chemotherapy and Cardiotoxicity
Cardiovasc Drugs Ther DOI 10.1007/s10557-016-6711-0 REVIEW ARTICLE Anthracycline Chemotherapy and Cardiotoxicity John V McGowan1 & Robin Chung1 & Angshuman Maulik1 & Izabela Piotrowska1 & JMalcolmWalker1 & Derek M Yellon1 # The Author(s) 2017. This article is published with open access at Springerlink.com Abstract Anthracycline chemotherapy maintains a promi- antibiotic overproduction techniques of the day [1]. nent role in treating many forms of cancer. Cardiotoxic side Anthracyclines are listed among the World Health effects limit their dosing and improved cancer outcomes ex- Organisation (WHO) model list of essential medicines [2]. pose the cancer survivor to increased cardiovascular morbidity Fifty years on from its discovery, anthracycline anti-tumour and mortality. The basic mechanisms of cardiotoxicity may and cardiotoxic mechanisms alike continue to evoke consider- involve direct pathways for reactive oxygen species genera- able interest in basic science and clinical trials research. tion and topoisomerase 2 as well as other indirect pathways. Cancer now affects more than one in three people in their Cardioprotective treatments are few and those that have been lifetime, and along with cardiovascular disease, they are the examined include renin angiotensin system blockade, beta two leading causes of death in developed nations. Overall ten- blockers, or the iron chelator dexrazoxane. New treatments year cancer survival stands at 50% across the twenty most exploiting the ErbB or other novel pro-survival pathways, common malignancies and approximately 80% or better in such as conditioning, are on the cardioprotection horizon. breast, lymphoma, melanoma and uterine cancers. These mor- Even in the forthcoming era of targeted cancer therapies, the tality trends reflect a broad improvement in survival rates in substantial proportion of today’s anthracycline-treated cancer the developed economies [3]. -
Acute Lymphoblastic Leukemia (ALL) (Part 1 Of
LEUKEMIA TREATMENT REGIMENS: Acute Lymphoblastic Leukemia (ALL) (Part 1 of 12) Note: The National Comprehensive Cancer Network (NCCN) Guidelines® for Acute Lymphoblastic Leukemia (ALL) should be consulted for the management of patients with lymphoblastic lymphoma. Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. They are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any -

TCRM-3694-Anthracycline Extravasation
Therapeutics and Clinical Risk Management Dovepress open access to scientific and medical research Open Access Full Text Article R EVIEW Anthracycline extravasation injuries: management with dexrazoxane Karin Jordan Abstract: The application of anthracyclines in anticancer therapy may result in accidental Timo Behlendorf extravasation injury and can be a serious complication of their use. Tissue necrosis with skin Franziska Mueller ulceration is a possible outcome in the inadvertent extravasation of anthracyclines during Hans-Joachim Schmoll intravenous administration. Until recently, there has been no effective treatment against the devastating effect of extravasated anthracycline. Preclinical and clinical studies are leading to Clinic for Internal Medicine IV, the clinical implementation of dexrazoxane as the first and only proven antidote in anthracycline Department for Oncology and Haematology, University extravasation. In two multicenter studies dexrazoxane has proven to be highly effective in Hospital Halle, Halle, Germany preventing skin necrosis and ulceration. This review focuses on the development and management of dexrazoxane in anthracycline extravasation injuries. Keywords: dexrazoxane, extravasation, necrosis, anthracyclines Introduction The extravasation of vesicant cancer chemotherapeutic agents, especially anthracyclines, remains one of the most distressing complications that hematologists and oncologists face in cytostatic, intravenous (iv) chemotherapy. Tissue necrosis with skin ulceration is a possible outcome in the -

(12) United States Patent (10) Patent No.: US 9,125,835 B2 Sinko Et Al
US009 125835B2 (12) United States Patent (10) Patent No.: US 9,125,835 B2 Sinko et al. (45) Date of Patent: Sep. 8, 2015 (54) SYNERGISTC COMBINATIONS TO REDUCE (56) References Cited PARTICLE DOSE FORTARGETED TREATMENT OF CANCER AND ITS U.S. PATENT DOCUMENTS METASTASES 6,294,204 B1* 9, 2001 Rossling et al. .............. 424/497 2005, 0043215 A1 2, 2005 Minko et al. (75) Inventors: Patrick J. Sinko, Annandale, NJ (US); 2005/0196343 A1* 9, 2005 Reddy et al. ............... 424,9322 Jieming Gao, New Brunswick, NJ (US); 2008/026O725 A1* 10, 2008 Naik et al. ................. 424,130.1 Manjeet Deshmukh, Edison, NJ (US); 2008/0280813 A1 11/2008 Minko et al. Xiaoping Zhang, Edison, NJ (US); 2008/031787O A1* 12, 2008 Ray et al. ...................... 424,649 Matthew S. Palombo, Marmora, NJ 2011/01 17024 A1 5, 2011 Sinko et al. (US); Sherif Ibrahim, Old Bridge, NJ 2011/0268803 A1 11, 2011 Prud’homme et al. (US) OTHER PUBLICATIONS (73) Assignee: RUTGERS, THE STATE UNIVERSITY OF NEWJERSEY, Linda Strandberg Ihrlund, et al., 3-Bromopyruvate as Inhibitor of New Brunswick, NJ (US) Tumor Cell Energy Metabolism and Chemopotentiator of Platinum Drugs, 2 Mol. Oncol. 94 (2008.* (*) Notice: Subject to any disclaimer, the term of this Ken Olaussen, etal, DNA Repair by ERCC1 in Non-Small-Cell Lung patent is extended or adjusted under 35 Cancer and Cisplatin-Based Adjuvant Chemotherapy, 355 N Engl. J U.S.C. 154(b) by 55 days. Med. 983 (2006).* Gang Ruan & Si-Shen Feng, Preparation and Characterization of (21) Appl. No.: 13/296,203 Poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG PLA) Microspheres for Controlled Release of Paclitaxel, 24 Biomat. -

Dexrazoxane Decreases the Cardiotoxic Effects of Doxorubicin in Osteosarcoma Patients Without Increasing Mortality from Secondary Malignant Neoplasms
Clinical Research in Practice: The Journal of Team Hippocrates Volume 7 Issue 1 Article 9 2021 Dexrazoxane decreases the cardiotoxic effects of doxorubicin in osteosarcoma patients without increasing mortality from secondary malignant neoplasms Thomas S. Przybycien Wayne State University, [email protected] Follow this and additional works at: https://digitalcommons.wayne.edu/crp Part of the Cardiology Commons, Medical Education Commons, Medical Pharmacology Commons, and the Oncology Commons Recommended Citation PRZYBYCIEN TS. Dexrazoxane decreases the cardiotoxic effects of doxorubicin in osteosarcoma patients without increasing mortality from secondary malignant neoplasms. Clin. Res. Prac. May 28 2021;7(1):eP2673. https://doi.org/10.22237/crp/1622160360 This Clinical Decision Report is brought to you for free and open access by the Open Access Journals at DigitalCommons@WayneState. It has been accepted for inclusion in Clinical Research in Practice: The Journal of Team Hippocrates by an authorized editor of DigitalCommons@WayneState. VOL 7 ISS 1 / eP2673 / MAY 28, 2021 https://doi.org/10.22237/crp/1622160360 Dexrazoxane decreases the cardiotoxic effects of doxorubicin in osteosarcoma patients without increasing mortality from secondary malignant neoplasms THOMAS S. PRZYBYCIEN, H. BSc., Wayne State University School of Medicine, [email protected] ABSTRACT A clinical decision report appraising: Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2016;63(1):54-615. https://doi.org/10.1002/pbc.25753 for a patient with osteosarcoma and concerns about the risk of secondary malignant neoplasms that attend use of dexarazoxane. -

4740350.Pdf (3.641Mb)
Network-based in silico drug efficacy screening The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Guney, Emre, Jörg Menche, Marc Vidal, and Albert-László Barábasi. 2016. “Network-based in silico drug efficacy screening.” Nature Communications 7 (1): 10331. doi:10.1038/ncomms10331. http:// dx.doi.org/10.1038/ncomms10331. Published Version doi:10.1038/ncomms10331 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26318623 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ARTICLE Received 7 May 2015 | Accepted 29 Nov 2015 | Published 1 Feb 2016 DOI: 10.1038/ncomms10331 OPEN Network-based in silico drug efficacy screening Emre Guney1,2,Jo¨rg Menche1,3, Marc Vidal2,4 & Albert-La´szlo´ Bara´basi1,2,3,5 The increasing cost of drug development together with a significant drop in the number of new drug approvals raises the need for innovative approaches for target identification and efficacy prediction. Here, we take advantage of our increasing understanding of the network-based origins of diseases to introduce a drug-disease proximity measure that quantifies the interplay between drugs targets and diseases. By correcting for the known biases of the interactome, proximity helps us uncover the therapeutic effect of drugs, as well as to distinguish palliative from effective treatments. Our analysis of 238 drugs used in 78 diseases indicates that the therapeutic effect of drugs is localized in a small network neighborhood of the disease genes and highlights efficacy issues for drugs used in Parkinson and several inflammatory disorders. -

1 Efficacy and Safety of Glucarpidase for Routine
EFFICACY AND SAFETY OF GLUCARPIDASE FOR ROUTINE USE AFTER HIGH DOSE METHOTREXATE IN PATIENTS WITH BONE SARCOMA Dr MARTHA PERISOGLOU UCL MD(RES) 1 DECLARATION I, Martha Perisoglou confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 ACKNOWLEDGEMENTS I feel privileged to have been able to work on and complete this doctoral thesis; I am deeply grateful to God and all who made this wonderful journey possible. I would like to express my sincerest appreciation and gratitude to the Richard Scowcroft Foundation for financially supporting my research. I would like to thank my supervisors Prof Jeremy Whelan and Prof John Hartley for their support, patience, guidance and constructive comments during the past years. I am particularly grateful to Janet Hartley for her mentorship, advice, care and friendship in the course of this task; Janet’s contribution has been invaluable. I would also like to thank Dr Joe Standing for his help with the pharmacokinetic analysis and Paul Bassett for his contribution in the statistical analysis of my data. I owe my deepest gratitude to all the patients and their families for their participation in the GLU 1 study; their attitude towards life and illness has been inspirational. Special thanks are due to my dearly beloved parents, Father Ioannis and Chrysanthi Perisoglou, and my siblings, Ellie, Maria, Giorgos and Manolis; without their love, prayers and constant presence I could not have completed this project. I am always grateful to my beloved husband Jonathan for his continuous support, encouragement and profound understanding, his endless humour, his love and belief in me.