Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-Induced Nausea and Vomiting in Children with Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya Et Al
US 2006.0024.365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya et al. (43) Pub. Date: Feb. 2, 2006 (54) NOVEL DOSAGE FORM (30) Foreign Application Priority Data (76) Inventors: Navin Vaya, Gujarat (IN); Rajesh Aug. 5, 2002 (IN)................................. 699/MUM/2002 Singh Karan, Gujarat (IN); Sunil Aug. 5, 2002 (IN). ... 697/MUM/2002 Sadanand, Gujarat (IN); Vinod Kumar Jan. 22, 2003 (IN)................................... 80/MUM/2003 Gupta, Gujarat (IN) Jan. 22, 2003 (IN)................................... 82/MUM/2003 Correspondence Address: Publication Classification HEDMAN & COSTIGAN P.C. (51) Int. Cl. 1185 AVENUE OF THE AMERICAS A6IK 9/22 (2006.01) NEW YORK, NY 10036 (US) (52) U.S. Cl. .............................................................. 424/468 (22) Filed: May 19, 2005 A dosage form comprising of a high dose, high Solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of Related U.S. Application Data immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of (63) Continuation-in-part of application No. 10/630,446, modified release active ingredient per unit is from 500 mg to filed on Jul. 29, 2003. 1500 mg, a process for preparing the dosage form. Patent Application Publication Feb. 2, 2006 Sheet 1 of 10 US 2006/0024.365A1 FIGURE 1 FIGURE 2 FIGURE 3 Patent Application Publication Feb. 2, 2006 Sheet 2 of 10 US 2006/0024.365A1 FIGURE 4 (a) 7 FIGURE 4 (b) Patent Application Publication Feb. 2, 2006 Sheet 3 of 10 US 2006/0024.365 A1 FIGURE 5 100 ov -- 60 40 20 C 2 4. -

Blood Thinners These Medications Require up to 2 Weeks of Discontinued Use Prior to a Myelogram Procedure
MEDICATIONS TO AVOID PRE & POST MYELOGRAMS Radiocontrast agents used in myelography have the ability to lower the seizure threshold and when used with other drugs that also have this ability, may lead to convulsions. Drugs that possess the ability to lower seizure threshold should be discontinued at least 48 hours prior to myelography if possible and should not be resumed until at least 24 hours post-procedure. Drugs are listed below. ***Always consult with your prescribing physician prior to stoppage of your medication. Phenothiazines Antidepressants MAO Inhibitors Acetophenazine (Tindal) Amitriptyline (Elavil) Furazolidone (Furoxone) Chlorpromazine (Thorazine) Amoxapine (Asendin) Isocarboxazid (Marplan) Promethazine (Phenergan) Bupropion *(Welbutrin, Zyban) Pargyline (Eutonyl) Ethopropazine (Parsidol) Clomipramine (Anafranil, Placil) Phenelzine (Nardil) Fluphenazine (Prolixin) Desipramine (Norapramin) Procarbazine (Matulane) Mesoridazine (Serentil) Doxepin (Sinequan) Tranlcypromine (Parnate) Methdilazine (Tacaryl) Imipramine (Tofranil) Perphenazine (Trilafon) Maprotiline (Ludiomil) CNS Stimulants Proclorperazine (Compazine) Nortriptyline (Pamelor) Amphetamines Promazine (Sparine) Protriptyline (Vivactil) Pemoline (Cylert) Promethazine (Phenergan) Trimipramine (Surmontil) Thiethylperazine (Torecan) Thioridazine (Mellaril) Combination Antidepressants Trifluoperazine (Stelazine) Amitriptyline + Chlordiazepoxide (Limbitrol DS) Triflupromazine (Vesprin) Amitriptyline + Perphenazine (Triavil) Largon Levoprome Miscellaneous Antipsychotics -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

CPT / HCPCS Code Drug Description Approximate Cost Share
The information listed here is for our most prevalent plan. The amount you pay for a covered drug will depend on your plan’s coverage. Please refer to your Medical Plan GTB for more information. To find out the cost of your drugs, please contact HMSA Customer Service at 1-800-776-4672. If you receive services from a nonparticipating provider, you are responsible for a copayment plus any difference between the actual charge and the eligible charge. Legend $0 = no cost share $ = $100 and under $$ = over $100 to $250 $$$ = over $250 to $500 $$$$ = over $500 to $1000 $$$$$ = over $1000 1 = Please call HMSA Customer Service 1-800-776-4672 for cost share information. 2 = The cost share for this drug is dependent upon the diagnosis. Please call HMSA Customer Service at 1-800-772-4672 for more information. 3 = Cost share information for these drugs is dependent upon the dose prescribed. Please call HMSA Customer Service at 1- 800-772-4672 for more information. CPT / HCPCS approximate Code Drug Description cost share J0129 Abatacept Injection $$$$ J0130 Abciximab Injection 3 J0131 Acetaminophen Injection $ J0132 Acetylcysteine Injection $ J0133 Acyclovir Injection $ J0135 Adalimumab Injection $$$$ J0153 Adenosine Inj 1Mg $ J0171 Adrenalin Epinephrine Inject $ J0178 Aflibercept Injection $$$ J0180 Agalsidase Beta Injection 3 J0200 Alatrofloxacin Mesylate 3 J0205 Alglucerase Injection 3 J0207 Amifostine 3 J0210 Methyldopate Hcl Injection 3 J0215 Alefacept 3 J0220 Alglucosidase Alfa Injection 3 J0221 Lumizyme Injection 3 J0256 Alpha 1 Proteinase Inhibitor -

2004 Guide to Psychiatric Drug Interactions Sheldon H
Av Referencein Pocketailable Format —pg. 59 Educational Review Primary Psychiatry. 2004;11(2):39-60 2004 Guide to Psychiatric Drug Interactions Sheldon H. Preskorn, MD, and David Flockhart, MD, PhD drug alone but is either more than or Focus Points less than what would normally be • In a drug-drug interaction (DDI), the presence of a second drug alters the expected for the specific dose ingested. nature, magnitude, or duration of the effect of a given dose of a first drug. “Altered duration” means that the These interactions can be therapeutic or adverse, planned or unintended, nature of the effect is reasonably the but are always determined by the pharmacodynamics and pharmacokinet- same as can be expected from the victim ics of the drugs involved rather than their therapeutic indication. drug alone, but the effect either is short- • The risk of unintended and untoward DDIs is increasing in concert with er or longer lived than would normally both the increasing number of pharmaceuticals available and the number of be expected for the dose given. patients on multiple medications. The goal of this guide is to provide a quick reference for prescribers about • To avoid adverse DDIs, the prescriber must keep in mind fundamental prin- some of the major psychiatric DDIs. ciples of pharmacology and good clinical management. Furthermore, it presents general con- • The prescriber must know all of the medications that the patient is taking cepts which can aid prescribers in and be able to use available knowledge about their pharmacodynamic and avoiding untoward DDIs when possible pharmacokinetic mechanisms to minimize the risk of untoward DDIs. -

Procedure Codes, Section 3
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN – PROCEDURE CODES SECTION 3 - DRUGS and DRUG ADMINISTRATION Physician – Procedure Codes , Section 3- Drugs and Drug Administration _____________________________________________________________________________ Table of Contents GENERAL RULES AND INFORMATION ............................................................2 MMIS DRUG MODIFIERS ....................................................................................4 DRUGS.................................................................................................................5 IMMUNE GLOBULINS...................................................................................................5 VACCINES, TOXOIDS ..................................................................................................5 HYDRATION, THERAPEUTIC, PROPHYLACTIC AND DIAGNOSTIC INJECTIONS AND INFUSIONS (EXCLUDES CHEMOTHERAPY) .....................................................8 DRUGS ADMINISTERED OTHER THAN ORAL METHOD.........................................10 CHEMOTHERAPY ADMINISTRATION.......................................................................19 CHEMOTHERAPY DRUGS.........................................................................................21 Version 2008 – 1 (5/15/2008) Page 1 of 23 Physician – Procedure Codes , Section 3- Drugs and Drug Administration _____________________________________________________________________________ GENERAL RULES AND INFORMATION 1. BY REPORT: A service that is rarely provided, unusual, variable, -
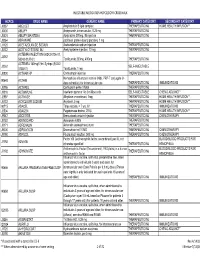
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Multiple Medications
Unless specifically noted, Medications need to be discontinued for 48 hours prior to exam and 24 hours post exam ALL OVER THE COUNTER SUPPLEMENTS OFF FOR 5 DAYS (examples: papaya enzymes, turmeric, cinnamon, vit. E, etc.) Abilify Asprin- 24 hours ConZip Abciximab- 24 hours Atomoxetine Coumadin- 5 days Acetophenazine Aventyl Cyclobenzaprine Actremra- 5 days Azilect Cycloserine Actron- 24 hours Bayer Asprin- 24 hours Cyclosporine Adapin Benzphentamine Cylert Adderall Bextra-24 hours Cymbalta Adipenx Bontril Dabigatran Advil- 24 hours Brilinta- 5 days Dalteparin- 5 days Aflaxin- 24 hours Brufen- 24 hours Daypro- 24 hours Aggrenox- 7 days Brupropion Dayrun- 24 hours Aleve- 24 hours Busiprone Daytrana Algix – 24 hours BuSpar Demerol Alka Seltzer- 24 hours Cambia-24 hours Desipramine Amantadine Cataflam- 24 hours Desoxyn Amitriptyline Ceeoxx- 24 hours Desvenlafaxine Amitriptyline + Chlordiazepoxide Celebrex- 24 hours Desyrel Amitriptyline + Perphenazine Celecoxib- 24 hours Dexedrine Amoxapine Celexa Dexketoprofen- 24 hours Amphetamine Ceoxx- 24 hours Dexmethylphenidate Amphetamine mixture Chlorpromazine Dextroamphetamine Amrix Chlorprothixene Diclofenac- 24 hours Anafranil Cilostazol- 5 days Didrex Anaprox- 24 hours Citalopram Diethylprophm Ansaid- 24 hours Clinoril- 24 hours Diflunisal- 24 hours Apixaban- Clomipramine Disalcid- 24 hours Aripiprazole Clopidogrel- 5 days Dividose Arixtra- 5 days Clopidogrel Bisulfate- 5 days Dolobid- 24 hours Armodafinil Clotam Rapid- 24 hours Dopram Arthrotec-24 hours Clozapine Doxapram Asaid-24 hours Clozaril -

Myelogram-Medications-To-Avoid.Pdf
Medication to be held 48 Banaflex /skeletal muscle relaxer Clozaril/antischizophenia HOURS BEFORE AND 24 HOURS Bancap/contains caffei Cogentin/antidyskinectic/ant AFTER MYELOGRAM Banzel/seizure adjunct iemetic/antipsychotic Updated December 2016 BC powder/ contains caffeine Compazine/phenothiazine Belviq/appetite suppressant Concerta/CNS stimulant Abilify / antipsychotic Bevespi contains formoterol Contrave/antidepressant Acetophenazine/ antipsychotic Benzphetamine/appetite Cyclizine/ antiemetic Adderall/amphetamine and suppressant antivertigo agent dextroamphetamine Bismuth subcitrate potassium/ Cyclobenzaprine/muscle Adipex-P/stimulant/ anorectic mineral relaxer Adsuva/antidepressant Bonine/meclicine Cylert/ADHD agent Adphen/ /antidepressant Bontril/appetite suppressant Cymbalta/antidepressant/tri appetite suppressant Bontril PDM/appetite suppressant cyclic Adipost/ appetite suppressants, Bortezomib/antineoplastics Sympathomimetic (Systemic Brintxelli / antidepressant Adrafinil/cognitive enhancer Bucladin-S softab/antiemetic Dapex/appetite suppressant Alatrofloxacin/ antibacterial antivertigo Dayquil/contains caffeine Aliskiren Bucet/contains caffeine Darvon 65/contain caffeine Hemifumarate/antihypertensive Buclizine/antiemetic/antivertigo Daytrana/CNS stimulant direct renal inhibitor / Budeption/antidepressant Desipramine/tricyclic/antide Alsuma/s Buphenamine/appetite suppressant pressant Amaphen/ barbituate Buproban/antidepressantr Desoxyn/amphetamine Amitriptyline/ tricyclic BupropionBuffets/caffeine Desvenlafaxine succinate/ antidepressant -

TEXAS RACING COMMISSION January 28, 2021 To
TEXAS RACING COMMISSION P. O. Box 12080, Austin, Texas 78711-2080 8505 Cross Park Drive, Suite 110, Austin, Texas 78754-4552 Phone (512) 833-6699 Fax (512) 833-6907 www.txrc.texas.gov January 28, 2021 To: Stewards, Commission Veterinarians, Test Barn Supervisors, Practicing Veterinarians, Owners, and Trainers From: Chuck Trout, Executive Director Re: Effective February 25, 2021 changes to the following documents: • Permissible Levels of Therapeutic Medications and Naturally Occurring Substances • Equine Medication Classification Policy and Penalty Guidelines • Equine Medication Classification List. This memo is to provide notice that the above listed documents are to be replaced effective this date. The changes include, but are not limited to: • Changes to the list of Permissible Levels of Therapeutic Medications and Naturally Occurring Substances; • Changes to the Equine Medication Classification Policy and Penalty Guidelines; • Changes to the Equine Medication Classification List. These documents are subject to further revision at any time. Test Barn Supervisors - please post this memo and the revised documents in the test barn as soon as possible. Also, please distribute copies of the Permissible Levels of Therapeutic Medications and Naturally Occurring Substances and Equine Medication Classification List to the practicing veterinarians at your racetrack. Licensing Staff - please post this memo and the revised documents where they may be viewed by the public as soon as possible. Copies of these documents will be made available on the Commission's website at http://www.txrc.texas.gov. Attachments: Permissible Levels of Therapeutic Medications and Naturally Occurring Substances Equine Medication Classification Policy and Penalty Guidelines Equine Medication Classification List TEXAS RACING COMMISSION P. -

New Information of Dopaminergic Agents Based on Quantum Chemistry Calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A
www.nature.com/scientificreports OPEN New information of dopaminergic agents based on quantum chemistry calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A. Ibarra3, Rubicelia Vargas4, Elisabeth Winnberg5 & Ana Martínez6* Dopamine is an important neurotransmitter that plays a key role in a wide range of both locomotive and cognitive functions in humans. Disturbances on the dopaminergic system cause, among others, psychosis, Parkinson’s disease and Huntington’s disease. Antipsychotics are drugs that interact primarily with the dopamine receptors and are thus important for the control of psychosis and related disorders. These drugs function as agonists or antagonists and are classifed as such in the literature. However, there is still much to learn about the underlying mechanism of action of these drugs. The goal of this investigation is to analyze the intrinsic chemical reactivity, more specifcally, the electron donor–acceptor capacity of 217 molecules used as dopaminergic substances, particularly focusing on drugs used to treat psychosis. We analyzed 86 molecules categorized as agonists and 131 molecules classifed as antagonists, applying Density Functional Theory calculations. Results show that most of the agonists are electron donors, as is dopamine, whereas most of the antagonists are electron acceptors. Therefore, a new characterization based on the electron transfer capacity is proposed in this study. This new classifcation can guide the clinical decision‑making process based on the physiopathological knowledge of the dopaminergic diseases. During the second half of the last century, a movement referred to as the third revolution in psychiatry emerged, directly related to the development of new antipsychotic drugs for the treatment of psychosis.