TEXAS RACING COMMISSION January 28, 2021 To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2021 List of Pharmacological Classes of Doping Agents and Doping Methods
BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 1 von 23 The 2021 list of pharmacological classes of doping agents and doping methods www.ris.bka.gv.at BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 2 von 23 www.ris.bka.gv.at BGBl. III - Ausgegeben am 8. Jänner 2021 - Nr. 1 3 von 23 THE 2021 PROHIBITED LIST WORLD ANTI-DOPING CODE DATE OF ENTRY INTO FORCE 1 January 2021 Introduction The Prohibited List is a mandatory International Standard as part of the World Anti-Doping Program. The List is updated annually following an extensive consultation process facilitated by WADA. The effective date of the List is 1 January 2021. The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. Below are some terms used in this List of Prohibited Substances and Prohibited Methods. Prohibited In-Competition Subject to a different period having been approved by WADA for a given sport, the In- Competition period shall in principle be the period commencing just before midnight (at 11:59 p.m.) on the day before a Competition in which the Athlete is scheduled to participate until the end of the Competition and the Sample collection process. Prohibited at all times This means that the substance or method is prohibited In- and Out-of-Competition as defined in the Code. Specified and non-Specified As per Article 4.2.2 of the World Anti-Doping Code, “for purposes of the application of Article 10, all Prohibited Substances shall be Specified Substances except as identified on the Prohibited List. -

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Meat Biotechnology – Applications in Pork Quality Muscle Targeted Growth Promotants – Mode of Action of Beta Agonists
Meat Biotechnology – Applications in Pork Quality Muscle Targeted Growth Promotants – Mode of Action of Beta Agonists Deana Hancock, Diane Moody, Dave Anderson Elanco Animal Health Reciprocal Meat Conference, June 19, 2006 Pharmacological Activity: Beta•Adrenergic Agonist What is a beta•adrenergic agonist? A beta•adrenergic agonist (b•agonist) binds to and activates beta•adrenergic receptors (b•AR) that are found on the surface of many different types of cells in the body. G A G M L V L V G P A A A L P D G Beta Adrenergic P G L E N S S A S A T A N D P V L R A Y Receptors L L P L C K P A S P S R C P A P R C A A D S E F E S D E Y G V R E P W S S E P L T H L S R F E Q Q N F V W G F A R A P T W C R A K D A E L W Y V R G V V I W A V L (1) M (3) W H (5) I N (7) F G T T S L A L L G A S L M A F V M F V S F F A D I N L P V V P V F V L Shaded AA's are V I L C L F S V L P L W Transmembrane L G L S F V T Y W G conserved across the L I M A V L V P C Y A Domains V L A L V E I S S G A S A D A I S I C L V F T N human b1, b2 and b3 T L M A N A L W V A G M F AR's (31%) V L C V T F I N V M S C V I P I I I V I V Y G A I A L L L I A (2) F L L (4) G R (6) T Y K N D R V K C A L R F A R T T G L L C Y R R K S Q C A R R P L A F A R L E Q P D F R K A L T Q A R A E A T Q R R I K P H T L L S A R R D G A H R R T Q R L V A G L V S C Circled AA's are S K P R A L S P P Q K D conserved across F R Y I R A R G D D D D R P D V V G A D K G G N P P S the b AR of S A P G A S T 1 C A E L P humans, pigs, R L L P R P P E A F G N W R and sheep (80%) L A A A A G C G A G G A A A R P A A T D P G P P P S S S L E S K A P D D E P A S V C R F R P P G A P P P S P S P S V A Mersmann, 1998; J. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Drug Testing Program
DRUG TESTING PROGRAM Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING CONTENTS 1. DRUG-FREE COMPETITION 2. ATHLETE CONSENT 3. DRUG TESTING 4. IN-COMPETITION/OUT-OF-COMPETITION DRUG TESTING 5. REGISTERED ATHLETE TESTING POOL (OUT-OF-COMPETITION DRUG TESTING) 6. REMOVAL FROM TESTING POOL/RETIREMENT 6A. REMOVAL FROM TESTING POOL/WATCH LIST 7. TESTING POOL REQUIREMENTS FOLLOWING A SANCTION 8. DRUG TEST NOTIFICATION AND ADMINISTRATION 9. SPECIMEN ANALYSIS 10. REPORTING RESULTS 11. DRUG TESTING POLICY VIOLATIONS 12. ENFORCEMENT/SANCTIONS 13. APPEALS PROCESS 14. LEADERBOARD DISPLAY 15. EDUCATION 16. DIETARY SUPPLEMENTS 17. TRANSGENDER POLICY 18. THERAPEUTIC USE EXEMPTION APPENDIX A: 2020-2021 CROSSFIT BANNED SUBSTANCE CLASSES APPENDIX B: CROSSFIT URINE TESTING PROCEDURES - (IN-COMPETITION) APPENDIX C: TUE APPLICATION REQUIREMENTS Drug Testing Policy V4 Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. [ 2 ] 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING 1. DRUG-FREE COMPETITION As the world’s definitive test of fitness, CrossFit Games competitions stand not only as testaments to the athletes who compete but to the training methodologies they use. In this arena, a true and honest comparison of training practices and athletic capacity is impossible without a level playing field. Therefore, the use of banned performance-enhancing substances is prohibited. Even the legal use of banned substances, such as physician-prescribed hormone replacement therapy or some over-the-counter performance-enhancing supplements, has the potential to compromise the integrity of the competition and must be disallowed. With the health, safety, and welfare of the athletes, and the integrity of our sport as top priorities, CrossFit, LLC has adopted the following Drug Testing Policy to ensure the validity of the results achieved in competition. -

United States Patent (10) Patent No.: US 8,916,581 B2 Boyd Et Al
USOO891 6581 B2 (12) United States Patent (10) Patent No.: US 8,916,581 B2 Boyd et al. (45) Date of Patent: *Dec. 23, 2014 (54) (S)-N-METHYLNALTREXONE 4,194,045 A 3, 1980 Adelstein 4,203,920 A 5, 1980 Diamond et al. (75) Inventors: Thomas A. Boyd, Grandview, NY (US); 4,241,066 A 12, 1980 Kobylecki et al. H OW d Wagoner,goner, Warwick,s NY (US);s 4,311,833.4,277,605 A T.1/1982 1981 NamikoshiBuyniski et etal. al. Suketu P. Sanghvi, Kendall Park, NJ 4.322,426 A 3/1982 Hermann et al. (US); Christopher Verbicky, 4.326,074 A 4, 1982 Diamond et al. Broadalbin, NY (US); Stephen 4.326,075 A 4, 1982 Diamond et al. “. s 4,377.568 A 3/1983 Chopra et al. Andruski, Clifton Park, NY (US) 4.385,078 A 5/1983 Onda et al. 4.427,676 A 1/1984 White et al. (73) Assignee: Progenics Pharmaceuticals, Inc., 4,430,327 A 2, 1984 Frederickson et al. Tarrytown, NY (US) 4,452,775 A 6/1984 Kent 4,457,907 A 7/1984 Porteret al. (*) Notice: Subject to any disclaimer, the term of this 4,462.839 A 7/1984 McGinley et al. patent is extended or adjusted under 35 4,518.4334,466,968 A 5/19858, 1984 McGinleyBernstein et al. U.S.C. 154(b) by 344 days. 4,533,739 A 8/1985 Pitzele et al. This patent is Subject to a terminal dis- 4,606,9094,556,552 A 12/19858/1986 PorterBechgaard et al. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
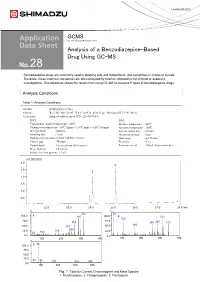
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Disposition of T Oxic Drugs and Chemicals
Disposition of Toxic Drugs and Chemicals in Man, Eleventh Edition Eleventh Edition in Man and Chemicals Drugs Toxic Disposition of The purpose of this work is to present in a single convenient source the current essential information on the disposition of the chemi- cals and drugs most frequently encountered in episodes of human poisoning. The data included relate to the body fluid concentrations of substances in normal or therapeutic situations, concentrations in fluids and tissues in instances of toxicity and the known metabolic fate of these substances in man. Brief mention is made of specific analytical procedures that are applicable to the determination of each substance and its active metabolites in biological specimens. It is expected that such information will be of particular interest and use to toxicologists, pharmacologists, clinical chemists and clinicians who have need either to conduct an analytical search for these materials in specimens of human origin or to interpret 30 Amberwood Parkway analytical data resulting from such a search. Ashland, OH 44805 by Randall C. Baselt, Ph.D. Former Director, Chemical Toxicology Institute Bookmasters Foster City, California HARD BOUND, 7” x 10”, 2500 pp., 2017 ISBN 978-0-692-77499-1 USA Reviewer Comments on the Tenth Edition “...equally useful for clinical scientists and poison information centers and others engaged in practice and research involving drugs.” Y. Caplan, J. Anal. Tox. “...continues to be an invaluable and essential resource for the forensic toxicologist and pathologist.” D. Fuller, SOFT ToxTalk “...has become an essential reference book in many laboratories that deal with clinical or forensic cases of poisoning.” M. -

The Effect of the Roots of Empathy Program on the Use of Psychotropic Medications Among Youth in Manitoba
The effect of the Roots of Empathy program on the use of psychotropic medications among youth in Manitoba by Lindsey Dahl A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of MASTER OF SCIENCE Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine University of Manitoba Winnipeg Copyright © 2017 by Lindsey Dahl Abstract Background: Psychotropic medications prescriptions to youth have increased. Roots of Empathy (ROE) is a social and emotional learning program that may influence the use of psychotropic medication. Methods: Administrative data was analyzed in a matched sample of children who received ROE during 2002/03 to 2012/13. Kaplan-Meier survival curves and Cox proportional hazard models were used to estimate the association between ROE and psychotropic medication dispensations. Results: Few significant differences were observed. Children who received ROE in kindergarten to grade 3 had a lower adjusted hazard for an anxiolytic dispensation. Children who received ROE in grade 7 to 8 had a higher hazard for an antipsychotic dispensation. Males who received the program had an increased hazard for an antipsychotic dispensation. Conclusion: There was no consistent differences in the likelihood of being dispensed a psychotropic medication between children who received ROE and children who did not in Manitoba. ii Acknowledgments I would like to first thank my thesis advisor Dr. Randy Fransoo of the Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine at the University of Manitoba. Right from our initial meeting, Dr.