Approved Cancer Drugs for Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
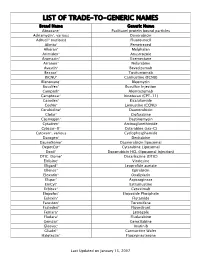
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Late Effects Among Long-Term Survivors of Childhood Acute Leukemia in the Netherlands: a Dutch Childhood Leukemia Study Group Report
0031-3998/95/3805-0802$03.00/0 PEDIATRIC RESEARCH Vol. 38, No.5, 1995 Copyright © 1995 International Pediatric Research Foundation, Inc. Printed in U.S.A. Late Effects among Long-Term Survivors of Childhood Acute Leukemia in The Netherlands: A Dutch Childhood Leukemia Study Group Report A. VAN DER DOES-VAN DEN BERG, G. A. M. DE VAAN, J. F. VAN WEERDEN, K. HAHLEN, M. VAN WEEL-SIPMAN, AND A. J. P. VEERMAN Dutch Childhood Leukemia Study Group,' The Hague, The Netherlands A.8STRAC ' Late events and side effects are reported in 392 children cured urogenital, or gastrointestinal tract diseases or an increased vul of leukemia. They originated from 1193 consecutively newly nerability of the musculoskeletal system was found. However, diagnosed children between 1972 and 1982, in first continuous prolonged follow-up is necessary to study the full-scale late complete remission for at least 6 y after diagnosis, and were effects of cytostatic treatment and radiotherapy administered treated according to Dutch Childhood Leukemia Study Group during childhood. (Pediatr Res 38: 802-807, 1995) protocols (70%) or institutional protocols (30%), all including cranial irradiation for CNS prophylaxis. Data on late events (relapses, death in complete remission, and second malignancies) Abbreviations were collected prospectively after treatment; late side effects ALL, acute lymphocytic leukemia were retrospectively collected by a questionnaire, completed by ANLL, acute nonlymphocytic leukemia the responsible pediatrician. The event-free survival of the 6-y CCR, continuous first complete remission survivors at 15 y after diagnosis was 92% (±2%). Eight late DCLSG, Dutch Childhood Leukemia Study Group relapses and nine second malignancies were diagnosed, two EFS, event free survival children died in first complete remission of late toxicity of HR, high risk treatment, and one child died in a car accident. -

The Effects of Pediatric Acute Lymphoblastic Leukemia on Social Competence: an Investigation Into the First Three Months of Treatment
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2010 The Effects of Pediatric Acute Lymphoblastic Leukemia on Social Competence: An Investigation into the First Three Months of Treatment Rachel L. Duchoslav Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Clinical Psychology Commons Recommended Citation Duchoslav, Rachel L., "The Effects of Pediatric Acute Lymphoblastic Leukemia on Social Competence: An Investigation into the First Three Months of Treatment" (2010). All Graduate Theses and Dissertations. 549. https://digitalcommons.usu.edu/etd/549 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. THE EFFECTS OF PEDIATRIC ACUTE LYMPHOBLASTIC LEUKEMIA ON SOCIAL COMPETENCE: AN INVESTIGATION INTO THE FIRST THREE MONTHS OF TREATMENT by Rachel L. Duchoslav A thesis submitted in partial fulfillment of the requirement for the degree of MASTER OF SCIENCE in Psychology Approved: Clinton E. Field, Ph.D. J. Dennis Odell, M.D. Major Professor Committee Member M. Scott DeBerard, Ph. D. Byron R. Burnham, Ed.D. Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2010 ii Copyright © Rachel L. Duchoslav 2010 All rights reserved iii ABSTRACT The Effects of Pediatric Acute Lymphoblastic Leukemia on Social Competence: An Investigation into the First Three Months of Treatment by Rachel L. Duchoslav, Master of Science Utah State University, 2010 Major Professor: Clinton E. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Health | Childhood Cancer America's Children and the Environment
Health | Childhood Cancer Childhood Cancer Cancer is not a single disease, but includes a variety of malignancies in which abnormal cells divide in an uncontrolled manner. These cancer cells can invade nearby tissues and can migrate by way of the blood or lymph systems to other parts of the body.1 The most common childhood cancers are leukemias (cancers of the white blood cells) and cancers of the brain or central nervous system, which together account for more than half of new childhood cancer cases.2 Cancer in childhood is rare compared with cancer in adults, but still causes more deaths than any factor, other than injuries, among children from infancy to age 15 years.2 The annual incidence of childhood cancer has increased slightly over the last 30 years; however, mortality has declined significantly for many cancers due largely to improvements in treatment.2,3 Part of the increase in incidence may be explained by better diagnostic imaging or changing classification of tumors, specifically brain tumors.4 However, the President’s Cancer Panel recently concluded that the causes of the increased incidence of childhood cancers are not fully understood, and cannot be explained solely by the introduction of better diagnostic techniques. The Panel also concluded that genetics cannot account for this rapid change. The proportion of this increase caused by environmental factors has not yet been determined.5 The causes of cancer in children are poorly understood, though in general it is thought that different forms of cancer have different causes. According to scientists at the National Cancer Institute, established risk factors for the development of childhood cancer include family history, specific genetic syndromes (such as Down syndrome), high levels of radiation, and certain pharmaceutical agents used in chemotherapy.4,6 A number of studies suggest that environmental contaminants may play a role in the development of childhood cancers. -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Biology and Disease Associations of Epstein±Barr Virus
doi 10.1098/rstb.2000.0783 Biology and disease associations of Epstein±Barr virus Dorothy H. Crawford Division of Biomedical and Clinical Laboratory Sciences, Edinburgh University Medical School,Teviot Place, Edinburgh EH89AG, UK ([email protected]) Epstein^Barr virus (EBV) is a human herpesvirus which infects almost all of the world's population subclinically during childhood and thereafter remains in the body for life. The virus colonizes antibody- producing (B) cells, which, as relatively long-lived resting cells, are an ideal site for long-term residence. Here EBV evades recognition and destruction by cytotoxic Tcells. EBV is passed to naive hosts in saliva, but how the virus gains access to this route of transmission is not entirely clear. EBVcarries a set of latent genes that, when expressed in resting B cells, induce cell proliferation and thereby increase the chances of successful virus colonization of the B-cell system during primary infection and the establishment of persis- tence. However, if this cell proliferation is not controlled, or if it is accompanied by additional genetic events within the infected cell, it can lead to malignancy. Thus EBV acts as a step in the evolution of an ever-increasing list of malignancies which are broadly of lymphoid or epithelial cell origin. In some of these, such as B-lymphoproliferative disease in the immunocompromised host, the role of the virus is central and well de¢ned; in others, such as Burkitt's lymphoma, essential cofactors have been identi¢ed which act in concert with EBV in the evolution of the malignant clone. -

Interplay Between Epstein-Barr Virus Infection and Environmental Xenobiotic Exposure in Cancer Francisco Aguayo1* , Enrique Boccardo2, Alejandro Corvalán3, Gloria M
Aguayo et al. Infectious Agents and Cancer (2021) 16:50 https://doi.org/10.1186/s13027-021-00391-2 REVIEW Open Access Interplay between Epstein-Barr virus infection and environmental xenobiotic exposure in cancer Francisco Aguayo1* , Enrique Boccardo2, Alejandro Corvalán3, Gloria M. Calaf4,5 and Rancés Blanco6 Abstract Epstein-Barr virus (EBV) is a herpesvirus associated with lymphoid and epithelial malignancies. Both B cells and epithelial cells are susceptible and permissive to EBV infection. However, considering that 90% of the human population is persistently EBV-infected, with a minority of them developing cancer, additional factors are necessary for tumor development. Xenobiotics such as tobacco smoke (TS) components, pollutants, pesticides, and food chemicals have been suggested as cofactors involved in EBV-associated cancers. In this review, the suggested mechanisms by which xenobiotics cooperate with EBV for carcinogenesis are discussed. Additionally, a model is proposed in which xenobiotics, which promote oxidative stress (OS) and DNA damage, regulate EBV replication, promoting either the maintenance of viral genomes or lytic activation, ultimately leading to cancer. Interactions between EBV and xenobiotics represent an opportunity to identify mechanisms by which this virus is involved in carcinogenesis and may, in turn, suggest both prevention and control strategies for EBV-associated cancers. Keywords: Epstein-Barr virus, environmental, cancer Introduction persistently infects approximately 90% of the world Approximately 13% of the cancer burden worldwide is population [5]. This virus establishes latent persistent in- etiologically related to viral infections with variations de- fections in B cells and is transmitted via nasopharyngeal pending on sociodemographic factors [1, 2]. The long- secretions [6]. -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

A Brain Tumor Epidemiology Consortium Review
Published OnlineFirst September 5, 2014; DOI: 10.1158/1055-9965.EPI-14-0207 Cancer Epidemiology, Review Biomarkers & Prevention Childhood Brain Tumor Epidemiology: A Brain Tumor Epidemiology Consortium Review Kimberly J. Johnson1, Jennifer Cullen2, Jill S. Barnholtz-Sloan3, Quinn T. Ostrom3, Chelsea E. Langer4,5,6, Michelle C. Turner4,5,6,7, Roberta McKean-Cowdin8, James L. Fisher9, Philip J. Lupo10,11, Sonia Partap12, Judith A. Schwartzbaum9, and Michael E. Scheurer10,11 Abstract Childhood brain tumors are the most common pediatric solid tumor and include several histologic subtypes. Although progress has been made in improving survival rates for some subtypes, understanding of risk factors for childhood brain tumors remains limited to a few genetic syndromes and ionizing radiation to the head and neck. In this report, we review descriptive and analytical epidemiology childhood brain tumor studies from the past decade and highlight priority areas for future epidemiology investigations and methodological work that is needed to advance our understanding of childhood brain tumor causes. Specifically, we summarize the results of a review of studies published since 2004 that have analyzed incidence and survival in different international regions and that have examined potential genetic, immune system, developmental and birth characteristics, and environmental risk factors. Cancer Epidemiol Biomarkers Prev; 23(12); 2716–36. Ó2014 AACR. Introduction Descriptive Epidemiology Brain and central nervous system (CNS) tumors are the There are >100 different histologic subtypes of CNS most common solid tumor and the second leading cause tumors with the incidence of each varying by age and of cancer-related death in individuals 0 to 19 years of age histologic subtype. -

Next Steps After Treatment
cancer.org | 1.800.227.2345 After Neuroblastoma Treatment Living as a Neuroblastoma Survivor For many people, cancer treatment often raises questions about next steps as a survivor. ● What Happens After Treatment for Neuroblastoma? Cancer Concerns After Treatment Neuroblastoma survivors are at risk for possible late effects of their cancer treatment. It’s important to discuss what these possible effects might be with your child’s medical team so you know what to watch for and report to the doctor. ● Late and Long-Term Effects of Neuroblastoma and Its Treatment What Happens After Treatment for Neuroblastoma? During treatment for neuroblastoma, the main concerns for most families are the daily aspects of getting through treatment and beating the cancer. After treatment, the concerns tend to shift toward the long-term effects of neuroblastoma and its treatment, as well as worries about neuroblastoma coming back. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 It's certainly normal to want to put neuroblastoma and its treatment behind you and to get back to a life that doesn’t revolve around cancer. But getting the right follow-up care offers your child the best chance for recovery and long-term survival. Follow-up exams and tests After treatment, the doctor will probably order follow-up tests, which may include lab tests and imaging tests1 (MIBG scans, PET scans, ultrasound, CT scans, and/or MRI scans) to see if there is any tumor remaining. The tests done will depend on the child's risk group2, the size and location of the tumor, and other factors.