Febuxostat for Management of Tumor Lysis Syndrome Including Its Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
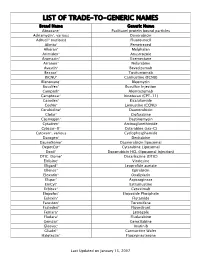
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Effects of Febuxostat on Autistic Behaviors and Computational Investigations of Diffusion and Pharmacokinetics
Effects of Febuxostat on Autistic Behaviors and Computational Investigations of Diffusion and Pharmacokinetics Jamelle M. Simmons Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Engineering Yong W. Lee, Chair Luke E. Achenie Hampton C. Gabler Alexei Morozov Aaron S. Goldstein John H. Rossmeisl November 27, 2018 Blacksburg, Virginia Keywords: Autism Spectrum Disorder, Xanthine Oxidase, Reactive Oxygen Species, Pharmacokinetics, Animal Behavior Copyright 2019, Jamelle M. Simmons Effects of Febuxostat on Autistic Behaviors and Computational Investigations of Diffusion and Pharmacokinetics Jamelle M. Simmons (ABSTRACT) Autism spectrum disorder (ASD) is a lifelong disability that has seen a rise in prevalence from 1 in 150 children to 1 in 59 between 2000 and 2014. Patients show behavioral ab- normalities in the areas of social interaction, communication, and restrictive and repetitive behaviors. As of now, the exact cause of ASD is unknown and literature points to multiple causes. The work contained within this dissertation explored the reduction of oxidative stress in brain tissue induced by xanthine oxidase (XO). Febuxostat is a new FDA approved XO- inhibitor that has been shown to be more selective and potent than allopurinol in patients with gout.The first study developed a computational model to calculate an effective diffu- sion constant (Deff ) of lipophilic compounds, such as febuxostat, that can cross endothelial cells of the blood-brain barrier (BBB) by the transcellular pathway. In the second study, male juvenile autistic (BTBR) mice were treated with febuxostat for seven days followed by behavioral testing and quantification of oxidative stress in brain tissue compared to controls. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

ULORIC Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------WARNINGS AND PRECAUTIONS------------------- These highlights do not include all the information needed to use ULORIC safely and effectively. See full prescribing • Cardiovascular Death: In a CV outcomes study, there was a higher information for ULORIC. rate of CV death in patients treated with ULORIC compared to allopurinol; in the same study ULORIC was non-inferior to ULORIC (febuxostat) tablets, for oral use allopurinol for the primary endpoint of major adverse Initial U.S. Approval: 2009 cardiovascular events (MACE). Consider the risks and benefits of WARNING: CARDIOVASCULAR DEATH ULORIC when deciding to prescribe or continue patients on See full prescribing information for complete boxed warning. ULORIC. (1, 5.1) • Gout patients with established cardiovascular (CV) disease • Gout Flares: An increase in gout flares is frequently observed treated with ULORIC had a higher rate of CV death compared to during initiation of anti-hyperuricemic agents, including ULORIC. If those treated with allopurinol in a CV outcomes study. (5.1) a gout flare occurs during treatment, ULORIC need not be discontinued. Prophylactic therapy (i.e., non-steroidal anti- • Consider the risks and benefits of ULORIC when deciding to inflammatory drug [NSAID] or colchicine upon initiation of prescribe or continue patients on ULORIC. ULORIC should only treatment) may be beneficial for up to six months. (2.4, 5.2) be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to • Hepatic Effects: Postmarketing reports of hepatic failure, allopurinol, or for whom treatment with allopurinol is not sometimes fatal. Causality cannot be excluded. -

Gouric Insert Final
Dosage Recommendations in Patients with Renal Impairment and Hepatic are at risk for severe drug-induced liver injury and should not be restarted on febuxostat. Impairment For patients with lesser elevations of serum ALT or bilirubin and with an alternate USE IN SPECIAL POPULATIONS No dose adjustment is necessary when administering Gouric in patients with mild or probable cause, treatment with febuxostat can be used with caution. moderate renal impairment. Pregnancy The recommended dosage of Gouric is limited to 40 mg once daily in patients with severe Thyroid disorders US FDA Pregnancy Category C. There are no adequate and well-controlled studies in renal impairment. Increased TSH values (>5.5 μIU/mL) were observed in patients on long-term treatment pregnant women. Febuxostat should be used during pregnancy only if the potential No dose adjustment is necessary in patients with mild to moderate hepatic impairment. with febuxostat (5.5%) in the long term open label extension studies. Caution is required benefit justifies the potential risk to the fetus. when febuxostat is used in patients with altered of thyroid function. Uric Acid Level Nursing mother COMPOSITION Testing for the target serum uric acid level of less than 6 mg/dL may be performed as early Effects on ability to drive and use machines Febuxostat is excreted in the milk of rats. It is not known whether this drug is excreted in Gouric® 40mg Tablet as two weeks after initiating Gouric therapy. Somnolence, dizziness, paraesthesia and blurred vision have been reported with the use of human milk. Because many drugs are excreted in human milk, caution should be Each film-coated tablet contains: Febuxostat. -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -
(12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner Et Al
US 2013 0059868A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner et al. (43) Pub. Date: Mar. 7, 2013 (54) TREATMENT OF GOUT Publication Classification (75) Inventors: Jeffrey Miner, San Diego, CA (US); Jean-Luc Girardet, San Diego, CA (51) Int. Cl. (US); Barry D. Quart, Encinitas, CA A613 L/496 (2006.01) (US) A6IP 29/00 (2006.01) (73) Assignee: Ardea Biociences, Inc., San Diego, CA A6IP 9/06 (2006.01) (US) A613 L/426 (2006.01) A 6LX3/59 (2006.01) (21) Appl. No.: 13/637,343 (52) U.S. Cl. ...................... 514/262.1: 514/384: 514/365 (22) PCT Fled: Mar. 29, 2011 (86) PCT NO.: PCT/US11A3O364 (57) ABSTRACT S371 (c)(1), (2), (4) Date: Oct. 24, 2012 Sodium 2-(5-bromo-4-(4-cyclopropyl-naphthalen-1-yl)-4H Related U.S. Application Data 1,2,4-triazol-3-ylthio)acetate is described. In addition, phar (60) Provisional application No. 61/319,014, filed on Mar. maceutical compositions and uses Such compositions for the 30, 2010. treatment of a variety of diseases and conditions. Patent Application Publication Mar. 7, 2013 Sheet 1 of 10 US 2013/00598.68A1 FIGURE 1 S. C SOC 55000 40 S. s 3. s Patent Application Publication Mar. 7, 2013 Sheet 2 of 10 US 2013/00598.68A1 ~~~::CC©>???>©><!--->?©><??--~~~~~·%~~}--~~~~~~~~*~~~~~~~~·;--~~~~~~~~~;~~~~~~~~~~}--~~~~~~~~*~~~~~~~~;·~~~~~ |×.> |||—||--~~~~ ¿*|¡ MSU No IL-1ra MSU50 IL-1ra MSU 100 IL-1ra MSU500 IL-1ra cells Only No IL-1 ra Patent Application Publication Mar. 7, 2013 Sheet 3 of 10 US 2013/00598.68A1 FIGURE 3 A: 50000 40000 R 30000 2 20000 10000 O -7 -6 -5 -4 -3 Lesinurad (log)M B: Lesinurad (log)M Patent Application Publication Mar. -

Azathioprine-Induced Severe Anemia Potentiated by the Concurrent Use Of
PRACTICE | CASES CPD Azathioprine-induced severe anemia potentiated by the concurrent use of allopurinol Lorenzo Madrazo MD, Emily Jones MD, Cyrus C. Hsia MD n Cite as: CMAJ 2021 January 18;193:E94-7. doi: 10.1503/cmaj.201022 66-year-old man presented to the emergency depart- ment with a 2-week history of progressive weakness and KEY POINTS lethargy. Three months before presentation, he had • Severe anemia and myelosuppression are rare but serious beenA started on azathioprine therapy for immunoglobulin (Ig) complications of azathioprine that are more likely to occur at G4-related biliary disease. Comorbidities included hypertension, high doses or when potentiated by interactions with other peripheral vascular disease, type 2 diabetes mellitus, salivary drugs. gland fibrosis, hypothyroidism, gastresophageal reflux disease, • Xanthine oxidase inhibitors such as allopurinol or febuxostat hyperlipidemia, osteoarthritis and gout. The patient was taking increase the production of myelotoxic metabolites from azathioprine. azathioprine 200 mg once daily and had been taking allopurinol 100 mg once daily for several years to manage his gout. Other • Initiation of azathioprine should be accompanied by regular monitoring of a complete blood count with differential and liver medications included sitagliptin 100 mg once daily, gliclazide enzymes at least every 2 weeks during initial dose titration, and, 120 mg once daily, acetylsalicylic acid 81 mg once daily, once stable, at least every 3 months thereafter, as clinically extended-release metoprolol 200 mg once daily, ramipril 5 mg appropriate. once daily, atorvastatin 40 mg once daily, rabeprazole 20 mg twice daily, clonazepam 2 mg at bedtime, gabapentin 100 mg 3 times daily, venlafaxine 225 mg daily, vitamin D 1000 IU once Investigations for anemia included upper and lower endos- daily and ibuprofen 800 mg as needed. -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

Approved Cancer Drugs for Children
U.S. FOOD & DRUG li1 ADMINISTRATION Approved Cancer Drugs for Children Amy Barone, MD, MSCI March 15, 2019 Frequent Criticism: Too few drugs approved for pediatric cancer “Since 1980, only 4 drugs have been approved for the first instance for use in children.” - Coalition Against Childhood Cancer “In the last 20 years, only two new drugs have been approved that were specifically developed to treat children with cancer.” – St. Baldricks “Over the past 20 years, the FDA has approved about 190 new cancer treatments for adults but only three for children.” USA Today “Since 1980, fewer than 10 drugs have been developed for use in children with cancer. Only three drugs have been approved for use in children. Only four additional new drugs have been approved for use by both adults and children.” - National Pediatric Cancer Foundation “15 oncology drugs were approved by the FDA for pediatric use between 1948 and 2003.” – Managed Care “From 1980 to 2017, only 11 drugs (already approved in adults) have been approved to use in children with cancer” - Coalition Against Childhood Cancer 2 Question: How many drugs are FDA approved to treat pediatric cancer? • A: 11 • B: 34 • C: 4 • D: 15 3 “There’s no tragedy in life like the death of a child.” - Dwight D. Eisenhower 4 Antitoxin Contamination • Early 1900s – Animal anti-sera given to patients with cholera, typhoid, etc. • A Horse named “Jim” – Contaminated serum – Anti-toxin resulted in deaths of 13 children • Second incident – Contaminated smallpox vaccine killed 9 children Laws Enacted 1902 – Biologics Control Act 1906 – Pure Food and Drug Act 6 Elixir Sulfanilamide Tragedy O ' 7 Law Enacted The Food, Drug and Cosmetic (FDC) Act of 1938 8 Thalidomide T~~:••~ ~ . -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented.