Chapter 4: Tumor Lysis Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
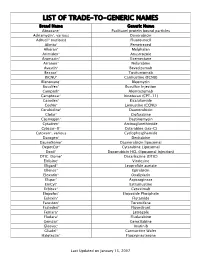
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

Approved Cancer Drugs for Children
U.S. FOOD & DRUG li1 ADMINISTRATION Approved Cancer Drugs for Children Amy Barone, MD, MSCI March 15, 2019 Frequent Criticism: Too few drugs approved for pediatric cancer “Since 1980, only 4 drugs have been approved for the first instance for use in children.” - Coalition Against Childhood Cancer “In the last 20 years, only two new drugs have been approved that were specifically developed to treat children with cancer.” – St. Baldricks “Over the past 20 years, the FDA has approved about 190 new cancer treatments for adults but only three for children.” USA Today “Since 1980, fewer than 10 drugs have been developed for use in children with cancer. Only three drugs have been approved for use in children. Only four additional new drugs have been approved for use by both adults and children.” - National Pediatric Cancer Foundation “15 oncology drugs were approved by the FDA for pediatric use between 1948 and 2003.” – Managed Care “From 1980 to 2017, only 11 drugs (already approved in adults) have been approved to use in children with cancer” - Coalition Against Childhood Cancer 2 Question: How many drugs are FDA approved to treat pediatric cancer? • A: 11 • B: 34 • C: 4 • D: 15 3 “There’s no tragedy in life like the death of a child.” - Dwight D. Eisenhower 4 Antitoxin Contamination • Early 1900s – Animal anti-sera given to patients with cholera, typhoid, etc. • A Horse named “Jim” – Contaminated serum – Anti-toxin resulted in deaths of 13 children • Second incident – Contaminated smallpox vaccine killed 9 children Laws Enacted 1902 – Biologics Control Act 1906 – Pure Food and Drug Act 6 Elixir Sulfanilamide Tragedy O ' 7 Law Enacted The Food, Drug and Cosmetic (FDC) Act of 1938 8 Thalidomide T~~:••~ ~ . -

At an Increased Risk: Tumor Lysis Syndrome
ONC O L O GY NURSING 101 DEBRA L. WINKELJ O HN , RN, MSN, AOCN®, CNS—ASS O CIATE ED IT O R At an Increased Risk: Tumor Lysis Syndrome Beth McGraw, RN, BSN, OCN® Patients at highest risk for tumor lysis hyperphosphatemia, hypocalcemia, and Gastrointestinal syndrome (TLS) often are diagnosed with hyperkalemia. Hyperuricemia occurs Hyperkalemia causes nausea, vomit- bulky, rapidly proliferating hematologic when the liver converts nucleic acids ing, and diarrhea (Cope, 2004). Anorexia, tumors, such as acute leukemia and non- into uric acid; hypocalcemia develops as abdominal cramping, and pain also may Hodgkin lymphoma (Kaplow & Hardin, serum calcium binds to elevated amounts occur because of the elevated potassium 2007). Patients with solid tumors, such of phosphorus within the bloodstream (McCance & Heuther, 2006). Decreased as mediastinal masses which are highly (Kaplow & Hardin). levels of serum calcium may cause in- sensitive to chemotherapy, also may de- testinal cramping and increased bowel velop TLS, although it is more common activity (McCance & Heuther). in patients undergoing treatment for leu- Clinical Findings kemia and lymphoma. TLS occurs from Laboratory findings will demonstrate the effect of chemotherapy or radiation electrolyte imbalances such as hyper- Cardiac on rapidly dividing cells. Patients with uricemia (more than 6.0 mg/dl), hyper- Serum potassium levels more than elevated lactic dehydrogenase (LDH), phosphatemia (more than 4.5 mg/dl), 5.3 mEq/l may cause irregular heart dehydration, and renal insufficiency are hypocalcemia (less than 8.5 mg/dl), and arrythmias and hypotension (Murphy- at greatest risk for developing TLS (Brant, hyperkalemia (more than 5.5 mEq/l) Ende & Chernecky, 2002). -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Clinical Characteristics of Tumor Lysis Syndrome in Childhood Acute Lymphoblastic Leukemia
www.nature.com/scientificreports OPEN Clinical characteristics of tumor lysis syndrome in childhood acute lymphoblastic leukemia Yao Xue1,2,22, Jing Chen3,22, Siyuan Gao1,2, Xiaowen Zhai5, Ningling Wang6, Ju Gao7, Yu Lv8, Mengmeng Yin9, Yong Zhuang10, Hui Zhang11, Xiaofan Zhu12, Xuedong Wu13, Chi Kong Li14, Shaoyan Hu15, Changda Liang16, Runming Jin17, Hui Jiang18, Minghua Yang19, Lirong Sun20, Kaili Pan21, Jiaoyang Cai3, Jingyan Tang3, Xianmin Guan4* & Yongjun Fang1,2* Tumor lysis syndrome (TLS) is a common and fatal complication of childhood hematologic malignancies, especially acute lymphoblastic leukemia (ALL). The clinical features, therapeutic regimens, and outcomes of TLS have not been comprehensively analyzed in Chinese children with ALL. A total of 5537 children with ALL were recruited from the Chinese Children’s Cancer Group, including 79 diagnosed with TLS. The clinical characteristics, treatment regimens, and survival of TLS patients were analyzed. Age distribution of children with TLS was remarkably diferent from those without TLS. White blood cells (WBC) count ≥ 50 × 109/L was associated with a higher risk of TLS [odds ratio (OR) = 2.6, 95% CI = 1.6–4.5]. The incidence of T-ALL in TLS children was signifcantly higher than that in non-TLS controls (OR = 4.7, 95% CI = 2.6–8.8). Hyperphosphatemia and hypocalcemia were more common in TLS children with hyperleukocytosis (OR = 2.6, 95% CI = 1.0–6.9 and OR = 5.4, 95% CI = 2.0–14.2, respectively). Signifcant diferences in levels of potassium (P = 0.004), calcium (P < 0.001), phosphorus (P < 0.001) and uric acid (P < 0.001) were observed between groups of TLS patients with and without increased creatinine. -

Management of Pediatric Tumor Lysis Syndrome
Arab Journal of Nephrology and Transplantation. 2011 Sep;4(3):147-54 Review Article AJNT Management of Pediatric Tumor Lysis Syndrome Illias tazi*¹, Hatim Nafil¹, Jamila Elhoudzi², Lahoucine Mahmal¹, Mhamed Harif² 1. Hematology department, Chu Mohamed VI, Cadi Ayyad University, Marrakech, Morocco 2. Hematology and Pediatric Oncology department, Chu Mohamed VI, Cadi Ayyad University, Marrakech, Morocco Abstract Keyswords: Acute Renal Failure; Burkitt’s Lymphoma; Hematologic Malignancies; Hydration; Tumor Lysis Introduction: Tumor lysis syndrome (TLS) is a serious Syndrome complication of malignancies and can result in renal failure or death. The authors declared no conflict of interest Review: In tumors with a high proliferative rate with a relatively large mass and a high sensitivity to cytotoxic Introduction agents, the initiation of therapy often results in the rapid release of intracellular anions, cations and the Infection remains the leading cause of organ failure in metabolic products of proteins and nucleic acids into critically ill cancer patients, but several reports point the bloodstream. The increased concentrations of uric out the increasing proportion of patients admitted into acid, phosphates, potassium and urea can overwhelm intensive care units with organ failure related to the the body’s homeostatic mechanisms to process and malignancy itself [1]. Some of these complications excrete these materials and result in the clinical spectrum may be directly related to the extent of the malignancy. associated with TLS. Typical clinical sequelae include This may include acute renal failure, acute respiratory gastrointestinal disturbances, neuromuscular effects, failure and coma. Most of these specific organ failures cardiovascular complications, acute renal failure and will require initiation of cancer therapy along with the death. -

Tumor Lysis Syndrome
Tumor Lysis Syndrome Thomas B. Russell, MD,* David E. Kram, MD, MCR* *Section of Pediatric Hematology/Oncology, Department of Pediatrics, Wake Forest School of Medicine, Winston-Salem, NC Practice Gaps Along with knowledge of how to evaluate a pediatric patient with a suspected malignancy, general pediatricians must maintain a high level of suspicion of tumor lysis syndrome for initial management and timely patient referral. This syndrome is largely preventable, and certainly manageable, with prompt diagnosis and appropriate intervention. Objectives After completing this article, readers should be able to: 1. Define and diagnose tumor lysis syndrome (TLS). 2. Recognize the risk factors for TLS. 3. Stratify pediatric patients with cancer according to risk of developing TLS. 4. Identify interventions to prevent TLS. 5. Discuss management strategies for patients with TLS. INTRODUCTION Tumor lysis syndrome (TLS) is a life-threatening oncologic emergency that occurs when cancer cells break down, either spontaneously or after initiation of cytotoxic chemo- therapy, and release their intracellular contents into the bloodstream. This massive release of uric acid, potassium, and phosphorous, which under normal physiologic conditions are excreted in the urine, can lead to hyperuricemia, hyperkalemia, hyper- phosphatemia, and hypocalcemia. These metabolic derangements increase the risk of severe complications, including acute kidney injury (AKI), cardiac arrhythmias, sei- zures, and even death. TLS is the most common oncologic emergency, and it occurs AUTHOR DISCLOSURE Drs Russell and Kram fi most frequently in children with acute leukemia and non-Hodgkin lymphoma. TLS is have disclosed no nancial relationships relevant to this article. This commentary does often preventable; clinicians must maintain a high index of suspicion and rely on not contain a discussion of an unapproved/ effective prevention and treatment strategies. -

A Systematic Review of Rhabdomyolysis for Clinical Practice Luis O
Chavez et al. Critical Care (2016) 20:135 DOI 10.1186/s13054-016-1314-5 REVIEW Open Access Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice Luis O. Chavez1, Monica Leon2, Sharon Einav3,4 and Joseph Varon5* Abstract Background: Rhabdomyolysis is a clinical syndrome that comprises destruction of skeletal muscle with outflow of intracellular muscle content into the bloodstream. There is a great heterogeneity in the literature regarding definition, epidemiology, and treatment. The aim of this systematic literature review was to summarize the current state of knowledge regarding the epidemiologic data, definition, and management of rhabdomyolysis. Methods: A systematic search was conducted using the keywords “rhabdomyolysis” and “crush syndrome” covering all articles from January 2006 to December 2015 in three databases (MEDLINE, SCOPUS, and ScienceDirect). The search was divided into two steps: first, all articles that included data regarding definition, pathophysiology, and diagnosis were identified, excluding only case reports; then articles of original research with humans that reported epidemiological data (e.g., risk factors, common etiologies, and mortality) or treatment of rhabdomyolysis were identified. Information was summarized and organized based on these topics. Results: The search generated 5632 articles. After screening titles and abstracts, 164 articles were retrieved and read: 56 articles met the final inclusion criteria; 23 were reviews (narrative or systematic); 16 were original articles containing epidemiological data; and six contained treatment specifications for patients with rhabdomyolysis. Conclusion: Most studies defined rhabdomyolysis based on creatine kinase values five times above the upper limit of normal. Etiologies differ among the adult and pediatric populations and no randomized controlled trials have been done to compare intravenous fluid therapy alone versus intravenous fluid therapy with bicarbonate and/or mannitol. -

Nebraska Medicine IV Push Medication Review
Nebraska Medicine IV Push Medication Review Adults Adults Pediatrics Pediatrics Can Med be Chemotherapy or If Med can be administered Can Med be If Med can be administered Drug Name administered IV Mab? IV push, how fast can it be administered IV IV push, how fast can it be push or < 5 pushed? push in? (Yes/No) pushed? min? (Yes/No) acetaZOLAMIDE No Yes 500 mg over 3 min Yes 500 mg over 3 min acetylcysteine No No No acyclovir No No No alteplase PE No No No alteplase stroke No No No amikacin No No No aminocaproic acid No No No aminophylline No No No amiodarone No No No ampho B (liposomal) No No No amphotericin B No No No ampicillin No Yes 500 mg over 3-5 min Yes 500 mg over 3-5 min ampicillin/sulbact No No No anthymocyte EQUINE No No No anthymocyte RABBI No No No anti-inhibitor FEIBA No No No antihem factor No Yes 5 min or less Yes 5 min or less antithrombin III No No No arginine No No No ascorbic acid No No No azithromycin No No No aztreonam No Yes 3 to 5 min Yes 3 to 5 min caffeine/sod benzoa No No No calcium chloride No No No calcium gluconate No No No ceFAZolin No Yes 3 to 5 min Yes 3 to 5 min cefePIME No Yes 5 min Yes 3 to 5 min cefoTAXime No Yes 3 to 5 min Yes 3 to 5 min cefOXitin No Yes 3 to 5 min Yes 3 to 5 min ceftaroline No No No cefTAZidime No Yes 3 to 5 min Yes 3 to 5 min cefTAZidime/avibacta No No No ceftolozane/tazobac No No No Nebraska Medicine IV Push Medication Review Adults Adults Pediatrics Pediatrics Can Med be Chemotherapy or If Med can be administered Can Med be If Med can be administered Drug Name administered IV