BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
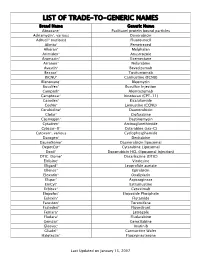
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Cyclophosphamide-Etoposide PO Ver
Chemotherapy Protocol LYMPHOMA CYCLOPHOSPHAMIDE-ETOPOSIDE ORAL Regimen Lymphoma – Cyclophosphamide-Etoposide PO Indication Palliative treatment of malignant lymphoma Toxicity Drug Adverse Effect Cyclophosphamide Dysuria, haemorrragic cystitis (rare), taste disturbances Etoposide Alopecia, hyperbilirubinaemia The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Patients diagnosed with Hodgkin’s Lymphoma carry a lifelong risk of transfusion associated graft versus host disease (TA-GVHD). Where blood products are required these patients must receive only irradiated blood products for life. Local blood transfusion departments must be notified as soon as a diagnosis is made and the patient must be issued with an alert card to carry with them at all times. Monitoring Drugs FBC, LFTs and U&Es prior to day one of treatment Albumin prior to each cycle Dose Modifications The dose modifications listed are for haematological, liver and renal function and drug specific toxicities only. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re-escalated in subsequent cycles without consultant approval. It is also a general rule for chemotherapy that if a third dose reduction is necessary treatment should be stopped. Please discuss all dose reductions / delays with the relevant consultant before prescribing, if appropriate. The approach may be different depending on the clinical circumstances. Version 1.1 (Jan 2015) Page 1 of 6 Lymphoma- Cyclophosphamide-Etoposide PO Haematological Dose modifications for haematological toxicity in the table below are for general guidance only. Always refer to the responsible consultant as any dose reductions or delays will be dependent on clinical circumstances and treatment intent. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Primary Effusion Lymphoma Without an Effusion: a Rare Case of Solid Extracavitary Variant of Primary Effusion Lymphoma in an HIV-Positive Patient
Hindawi Case Reports in Hematology Volume 2018, Article ID 9368451, 5 pages https://doi.org/10.1155/2018/9368451 Case Report Primary Effusion Lymphoma without an Effusion: A Rare Case of Solid Extracavitary Variant of Primary Effusion Lymphoma in an HIV-Positive Patient Hamza Hashmi ,1 Drew Murray,1 Samer Al-Quran ,2 and William Tse3 1Division of Hematology and Oncology, University of Louisville, Louisville, KY, USA 2Department of Pathology and Laboratory Medicine, University of Louisville, Louisville, KY, USA 3Division of Blood and Marrow Transplant, University of Louisville, Louisville, KY, USA Correspondence should be addressed to Hamza Hashmi; [email protected] Received 26 August 2017; Revised 5 December 2017; Accepted 27 December 2017; Published 28 January 2018 Academic Editor: Tatsuharu Ohno Copyright © 2018 Hamza Hashmi et al. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Primary effusion lymphoma (PEL) is a unique form of non-Hodgkin lymphoma, usually seen in severely immunocompromised, HIV-positive patients. PEL is related to human herpesvirus-8 (HHV-8) infection, and it usually presents as a lymphomatous body cavity effusion in the absence of a solid tumor mass. )ere have been very few case reports of HIV-positive patients with HHV-8- positive solid tissue lymphomas not associated with an effusion (a solid variant of PEL). In the absence of effusion, establishing an accurate diagnosis can be challenging, and a careful review of morphology, immunophenotype, and presence of HHV-8 is necessary to differentiate from other subtypes of non-Hodgkin lymphoma. -

Arsenic Trioxide Is Highly Cytotoxic to Small Cell Lung Carcinoma Cells
160 Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells 1 1 Helen M. Pettersson, Alexander Pietras, effect of As2O3 on SCLC growth, as suggested by an Matilda Munksgaard Persson,1 Jenny Karlsson,1 increase in neuroendocrine markers in cultured cells. [Mol Leif Johansson,2 Maria C. Shoshan,3 Cancer Ther 2009;8(1):160–70] and Sven Pa˚hlman1 1Center for Molecular Pathology, CREATE Health and 2Division of Introduction Pathology, Department of Laboratory Medicine, Lund University, 3 Lung cancer is the most frequent cause of cancer deaths University Hospital MAS, Malmo¨, Sweden; and Department of f Oncology-Pathology, Cancer Center Karolinska, Karolinska worldwide and results in 1 million deaths each year (1). Institute and Hospital, Stockholm, Sweden Despite novel treatment strategies, the 5-year survival rate of lung cancer patients is only f15%. Small cell lung carcinoma (SCLC) accounts for 15% to 20% of all lung Abstract cancers diagnosed and is a very aggressive malignancy Small cell lung carcinoma (SCLC) is an extremely with early metastatic spread (2). Despite an initially high aggressive form of cancer and current treatment protocols rate of response to chemotherapy, which currently com- are insufficient. SCLC have neuroendocrine characteristics bines a platinum-based drug with another cytotoxic drug and show phenotypical similarities to the childhood tumor (3, 4), relapses occur in the absolute majority of SCLC neuroblastoma. As multidrug-resistant neuroblastoma patients. At relapse, the efficacy of further chemotherapy is cells are highly sensitive to arsenic trioxide (As2O3) poor and the need for alternative treatments is obvious. in vitro and in vivo, we here studied the cytotoxic effects Arsenic-containing compounds have been used in tradi- of As2O3 on SCLC cells. -

Arsenic Trioxide Potentiates the Effectiveness of Etoposide in Ewing Sarcomas
INTERNATIONAL JOURNAL OF ONCOLOGY 49: 2135-2146, 2016 Arsenic trioxide potentiates the effectiveness of etoposide in Ewing sarcomas KAREN A. BOEHME1*, JULIANE NITSCH1*, ROSA RIESTER1, RUPert HANDGRETINGER2, SABINE B. SCHLEICHER2, TORSTEN KLUBA3,4 and FRANK TRAUB1,3 1Laboratory of Cell Biology, Department of Orthopaedic Surgery, Eberhard Karls University Tuebingen; 2Department of Haematology and Oncology, Children's Hospital, Eberhard Karls University Tuebingen; 3Department of Orthopaedic Surgery, Eberhard Karls University Tuebingen, Tuebingen; 4Department for Orthopaedic Surgery, Hospital Dresden-Friedrichstadt, Dresden, Germany Received June 12, 2016; Accepted July 28, 2016 DOI: 10.3892/ijo.2016.3700 Abstract. Ewing sarcomas (ES) are rare mesenchymal tumours, the drug concentrations used. With the exception of ATO in most commonly diagnosed in children and adolescents. Arsenic RD-ES cells, all drugs induced apoptosis in the ES cell lines, trioxide (ATO) has been shown to efficiently and selectively indicated by caspase-3 and PARP cleavage. Combination of target leukaemic blasts as well as solid tumour cells. Since the agents potentiated the reduction of viability as well as the multidrug resistance often occurs in recurrent and metastatic inhibitory effect on clonal growth. In addition, cell death induc- ES, we tested potential additive effects of ATO in combina- tion was obviously enhanced in RD-ES and SK-N-MC cells tion with the cytostatic drugs etoposide and doxorubicin. The by a combination of ATO and etoposide compared to single Ewing sarcoma cell lines A673, RD-ES and SK-N-MC as well application. Summarised, the combination of low dose, physi- as mesenchymal stem cells (MSC) for control were treated ologically easily tolerable ATO with commonly used etoposide with ATO, etoposide and doxorubicin in single and combined and doxorubicin concentrations efficiently and selectively application. -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

Approved Cancer Drugs for Children
U.S. FOOD & DRUG li1 ADMINISTRATION Approved Cancer Drugs for Children Amy Barone, MD, MSCI March 15, 2019 Frequent Criticism: Too few drugs approved for pediatric cancer “Since 1980, only 4 drugs have been approved for the first instance for use in children.” - Coalition Against Childhood Cancer “In the last 20 years, only two new drugs have been approved that were specifically developed to treat children with cancer.” – St. Baldricks “Over the past 20 years, the FDA has approved about 190 new cancer treatments for adults but only three for children.” USA Today “Since 1980, fewer than 10 drugs have been developed for use in children with cancer. Only three drugs have been approved for use in children. Only four additional new drugs have been approved for use by both adults and children.” - National Pediatric Cancer Foundation “15 oncology drugs were approved by the FDA for pediatric use between 1948 and 2003.” – Managed Care “From 1980 to 2017, only 11 drugs (already approved in adults) have been approved to use in children with cancer” - Coalition Against Childhood Cancer 2 Question: How many drugs are FDA approved to treat pediatric cancer? • A: 11 • B: 34 • C: 4 • D: 15 3 “There’s no tragedy in life like the death of a child.” - Dwight D. Eisenhower 4 Antitoxin Contamination • Early 1900s – Animal anti-sera given to patients with cholera, typhoid, etc. • A Horse named “Jim” – Contaminated serum – Anti-toxin resulted in deaths of 13 children • Second incident – Contaminated smallpox vaccine killed 9 children Laws Enacted 1902 – Biologics Control Act 1906 – Pure Food and Drug Act 6 Elixir Sulfanilamide Tragedy O ' 7 Law Enacted The Food, Drug and Cosmetic (FDC) Act of 1938 8 Thalidomide T~~:••~ ~ . -

Advances in Llm
LLM ADVANCES IN LLM Current Developments in the Management of Leukemia, Lymphoma, and Myeloma Section Editor: Susan O’Brien, MD Prophylaxis of Central Nervous System Relapse in Patients With Lymphoma Avyakta Kallam, MD Assistant Professor, Internal Medicine Division of Oncology & Hematology University of Nebraska Medical Center Omaha, Nebraska H&O How common is central nervous system AK Patients can be asymptomatic at the time of diag- relapse in lymphoma? nosis. When symptoms do occur, they can be subtle, and include nonspecific headaches, blurred vision, and AK The incidence of central nervous system (CNS) mild confusion. Overt symptoms include cranial nerve relapse in lymphoma depends on the type of disease. In palsies and focal neurologic deficits, such as weakness more-aggressive, high-grade lymphomas, such as Burkitt and imbalance. lymphoma and lymphoblastic lymphoma, the rate of Imaging studies, such as magnetic resonance imaging CNS relapse can reach 30% to 50%. In diffuse large of the brain, can help detect the presence of CNS involve- B-cell lymphoma, CNS involvement occurs in approxi- ment. The diagnostic test of choice, however, is lumbar mately 2% to 5% of patients. puncture with flow cytometry of the cerebral spinal fluid. H&O What are the risk factors? H&O Does CNS relapse impact prognosis? AK The validated Central Nervous System–International AK CNS relapse confers a poor prognosis in patients with Prognostic Index (CNS-IPI) scoring system is used to lymphoma, particularly diffuse large B-cell lymphoma. identify patients who are at high risk for CNS relapse. The median overall survival in a patient who develops The scoring system takes into account 6 characteristics: CNS relapse is approximately 3 to 4 months. -

Extracavitary/Solid Variant of Primary Effusion Lymphoma Yoonjung Kim, Mda,1, Vasiliki Leventaki, Mda, Feriyl Bhaijee, Mbchbb, ⁎ Courtney C
Available online at www.sciencedirect.com Annals of Diagnostic Pathology 16 (2012) 441–446 Extracavitary/solid variant of primary effusion lymphoma Yoonjung Kim, MDa,1, Vasiliki Leventaki, MDa, Feriyl Bhaijee, MBChBb, ⁎ Courtney C. Jackson, MDb, L. Jeffrey Medeiros, MDa, Francisco Vega, MD PhDa, aDepartment of Hematopathology, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA bDepartment of Pathology, University of Mississippi Medical Center, Jackson, MS, USA Abstract Primary effusion lymphoma (PEL) is a distinct clinicopathologic entity associated with human herpesvirus 8 (HHV8) infection that mostly affects patients with immunodeficiency. Primary effusion lymphoma usually presents as a malignant effusion involving the pleural, peritoneal, and/or pericardial cavities without a tumor mass. Rare cases of HHV8-positive lymphoma with features similar to PEL can present as tumor masses in the absence of cavity effusions and are considered to represent an extracavitary or solid variant of PEL. Here, we report 3 cases of extracavitary PEL arising in human immunodeficiency virus–infected men. Two patients had lymphadenopathy and underwent lymph node biopsy. One patient had a mass involving the ileum and ascending colon. In lymph nodes, the tumor was predominantly sinusoidal. The tumor involving the ileum and ascending colon presented as 2 masses, 12.5 × 10.6 × 2.6 cm in the colon and 3.6 × 2.7 × 1.9 cm in the ileum. In each case, the neoplasms were composed of large anaplastic cells, and 2 cases had “hallmark cells.” Immunohistochemistry showed that all cases were positive for HHV8 and CD138. One case also expressed CD4 and CD30, and 1 case was positive for Epstein-Barr virus–encoded RNA. -

Dose-Adjusted Epoch and Rituximab for the Treatment of Double
Dose-Adjusted Epoch and Rituximab for the treatment of double expressor and double hit diffuse large B-cell lymphoma: impact of TP53 mutations on clinical outcome by Anna Dodero, Anna Guidetti, Fabrizio Marino, Alessandra Tucci, Francesco Barretta, Alessandro Re, Monica Balzarotti, Cristiana Carniti, Chiara Monfrini, Annalisa Chiappella, Antonello Cabras, Fabio Facchetti, Martina Pennisi, Daoud Rahal, Valentina Monti, Liliana Devizzi, Rosalba Miceli, Federica Cocito, Lucia Farina, Francesca Ricci, Giuseppe Rossi, Carmelo Carlo-Stella, and Paolo Corradini Haematologica. 2021; Jul 22. doi: 10.3324/haematol.2021.278638 [Epub ahead of print] Received: February 26, 2021. Accepted: July 13, 2021. Citation: Anna Dodero, Anna Guidetti, Fabrizio Marino, Alessandra Tucci, Francesco Barretta, Alessandro Re, Monica Balzarotti, Cristiana Carniti, Chiara Monfrini, Annalisa Chiappella, Antonello Cabras, Fabio Facchetti, Martina Pennisi, Daoud Rahal, Valentina Monti, Liliana Devizzi, Rosalba Miceli, Federica Cocito, Lucia Farina, Francesca Ricci, Giuseppe Rossi, Carmelo Carlo-Stella, and Paolo Corradini. Dose-Adjusted Epoch and Rituximab for the treatment of double expressor and double hit d fuse large B-cell lymphoma: impact of TP53 mutations on clinical outcome. Publisher's Disclaimer. E-publishing ahead of print is increasingly important for the rapid dissemination of science. Haematologica is, therefore, E-publishing PDF files of an early version of manuscripts that have completed a regular peer review and have been accepted for publication. E-publishing of this PDF file has been approved by the authors. After having E-published Ahead of Print, manuscripts will then undergo technical and English editing, typesetting, proof correction and be presented for the authors' final approval; the final version of the manuscript will then appear in a regular issue of the journal. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole