Typha Angustifolia Management: Implications for Glacial Marsh Restoration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hybridization Dynamics of Invasive Cattail (Typhaceae) Stands at Pierce Cedar Creek Institute: a Molecular Analysis
HYBRIDIZATION DYNAMICS OF INVASIVE CATTAIL (TYPHACEAE) STANDS AT PIERCE CEDAR CREEK INSTITUTE: A MOLECULAR ANALYSIS Alex Graeff, Kelsey Huisman, and Dr. Pamela J. Laureto Department of Biological Sciences Grand Rapids Community College 143 Bostwick NE Grand Rapids, Michigan 49503 ABSTRACT Three cattail taxa are recognized in Michigan USA: native Typha latifolia (broad-leaf cattail), the invasive Typha angustifolia (narrow-leaf cattail), and the hybrid of these two species Typha × glauca. Typha angustifolia and T. × glauca are of special interest because of their ability to aggressively spread and out-compete the native cattail T. latifolia. Typha × glauca has been shown to out-compete both its parental taxa and produce monospecific stands. We surveyed the Pierce Cedar Creek Institute (PCCI) property for cattails and located 25 distinct cattail marshes. We determined the total area of cattail marsh at PCCI to be roughly 10% of the 267 ha property. Cattail individuals were sampled from each of the 25 stands and random amplified polymorphic DNA markers were used to identify the individuals to species. We found that 20 of the 25 stands were monospecific for the native cattail, T. latifolia. Five of the stands were mixtures of the native T. latifolia and the introduced T. angustifolia, and T. × glauca was found in two of the mixed stands. We recommend removal of the invasive T. angustifolia and T. × glauca individuals and the establishment of a monitoring plan in order to maintain the long-term health of the cattail marshes at PCCI. Keywords: Typha spp., RAPD markers, invasive species 1 INTRODUCTION Species of Typha L. (Typhaceae), commonly known as cattails, are highly productive emergent plants that grow in a variety of wetland habitats throughout the world (McManus et al. -

The Ayurvedic Pharmacopoeia of India
THE AYURVEDIC PHARMACOPOEIA OF INDIA PART- I VOLUME – V GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF AYUSH Contents | Monographs | Abbreviations | Appendices Legal Notices | General Notices Note: This e-Book contains Computer Database generated Monographs which are reproduced from official publication. The order of contents under the sections of Synonyms, Rasa, Guna, Virya, Vipaka, Karma, Formulations, Therapeutic uses may be shuffled, but the contents are same from the original source. However, in case of doubt, the user is advised to refer the official book. i CONTENTS Legal Notices General Notices MONOGRAPHS Page S.No Plant Name Botanical Name No. (as per book) 1 ËMRA HARIDRË (Rhizome) Curcuma amada Roxb. 1 2 ANISÍNA (Fruit) Pimpinella anisum Linn 3 3 A×KOLAH(Leaf) Alangium salviifolium (Linn.f.) Wang 5 4 ËRAGVËDHA(Stem bark) Cassia fistula Linn 8 5 ËSPHOÙË (Root) Vallaris Solanacea Kuntze 10 6 BASTËNTRÌ(Root) Argyreia nervosa (Burm.f.)Boj. 12 7 BHURJAH (Stem Bark) Betula utilis D.Don 14 8 CAÛÚË (Root) Angelica Archangelica Linn. 16 9 CORAKAH (Root Sock) Angelica glauca Edgw. 18 10 DARBHA (Root) Imperata cylindrica (Linn) Beauv. 21 11 DHANVAYËSAH (Whole Plant) Fagonia cretica Linn. 23 12 DRAVANTÌ(Seed) Jatropha glandulifera Roxb. 26 13 DUGDHIKË (Whole Plant) Euphorbia prostrata W.Ait 28 14 ELAVËLUKAê (Seed) Prunus avium Linn.f. 31 15 GAÛÚÌRA (Root) Coleus forskohlii Briq. 33 16 GAVEDHUKA (Root) Coix lachryma-jobi LInn 35 17 GHOÛÙË (Fruit) Ziziphus xylopyrus Willd. 37 18 GUNDRËH (Rhizome and Fruit) Typha australis -

Study of Structure and Properties of Tunisian Typha Leaf Fibers
International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181 Vol. 3 Issue 3, March - 2014 Study of Structure and Properties of Tunisian Typha Leaf Fibers Rezig Sana1, Jaouadi Mounir 2 , Msahli Slah3 1 PhD, Laboratory of Textile Engineering, University of Monastir, Tunisia 2 PhD, Laboratory of Textile Engineering, University of Monastir, Tunisia 3 Professor, Laboratory of Textile Engineering, University of Monastir, Tunisia Abstract —Typha fiber is derived from the leaves of Typha some researchers have tried to extract and develop Typha plant, which belongs to the family Typhaceae. The fiber has been fibers of different varieties in the world, the Tunisian Typha obtained using a hot alkali treatment. Some properties of this variety has not been described in the literature. fiber, such as diameter, linear density, tensile properties and thermal properties have been determined. In this study, we attempted to extract the Typha fibers and Using scanning electron microscope, the morphological structure remove non-cellulosic materials using a hot alkali treatment. of the obtained fibers has been investigated and shows a Fiber quality has been evaluated in terms of extraction yield, structure similar to a natural composite of ultimate fiber bundles diameter measurement, linear density, morphological and of cellulose. As a result, measured properties have been mechanical properties. Thermal analysis and Infrared spectrum compared with untreated typha fibers and other lignocellulosic have also been investigated, since fiber performance depends fibers in order to conclude on the importance of the textile potential of the Typha leaf fibers. on its chemical composition and physical properties. As a result, measured properties have been compared with untreated Keywords—Typha fiber; hot alkali treatment; thermal properties; Typha fibers and other lignocellulosic fibers in order to tensile properties; morphological structure. -

Potential Benefits and Toxicity of Nanoselenium and Nitric Oxide in Peppermint
doi:10.14720/aas.2018.111.2.11 Original research article / izvirni znanstveni članek Potential benefits and toxicity of nanoselenium and nitric oxide in peppermint Hossein NAZERIEH1, Zahra Oraghi ARDEBILI1*, Alireza IRANBAKHSH2 Received January 13, 2018; accepted May 04, 2018. Delo je prispelo 13. januarja 2018, sprejeto 04. maja 2018. ABSTRACT IZVLEČEK Taking account of nano-compounds and biofortification, this POTENCIALNE KORISTI IN STRUPENOST research was conducted to evaluate peppermint (Mentha x NANOSELENA IN DUŠIKOVEGA OKSIDA PRI piperita L.) responses to nano-selenium (nSe; 0, 2, and POPROVI METI 20 mg l-1) and/or nitric oxide (NO; 0 and 8 mg l-1). Significant increases in leaf length, and area, and shoot fresh mass were Raziskava je bila izvedena za ovrednostenje odziva poprove enhanced by the low level of nSe and/or NO, contrasted with mete (Mentha x piperita L.) na nano selen (nSe; 0, 2, in the high dose. The inhibitory effects of the high dose of nSe 20 mg l-1) in/ali dušikov oksid (NO; 0 in 8 mg l-1). Značilno on the growth-related characteristics were significantly povečanje dolžine in površine listov in sveže mase poganjkov mitigated by NO. The adverse impact of nSe20 on chlorophyll je bilo vzpodbujeno z majhnimi količinami nSe in/ali NO, concentration was alleviated by NO. The individual and nasprotno od učinkov velikih količin. Zaviralni učinki velikih combined treatments of nSe2 led to the significant inductions koncentracij nSe na z rastjo povezane parametre so bili in the activities of nitrate reductase and peroxidase, whereas značilno zmanjšani z dodatkom NO. -

ELEMENT STEWARDSHIP ABSTRACT for Typha Spp. North
ELEMENT STEWARDSHIP ABSTRACT for Typha spp. North American Cattails To the User: Element Stewardship Abstracts (ESAs) are prepared to provide The Nature Conservancy's Stewardship staff and other land managers with current management-related information on those species and communities that are most important to protect, or most important to control. The abstracts organize and summarize data from numerous sources including literature and researchers and managers actively working with the species or community. We hope, by providing this abstract free of charge, to encourage users to contribute their information to the abstract. This sharing of information will benefit all land managers by ensuring the availability of an abstract that contains up-to-date information on management techniques and knowledgeable contacts. Contributors of information will be acknowledged within the abstract and receive updated editions. To contribute information, contact the editor whose address is listed at the end of the document. For ease of update and retrievability, the abstracts are stored on computer at the national office of The Nature Conservancy. This abstract is a compilation of available information and is not an endorsement of particular practices or products. Please do not remove this cover statement from the attached abstract. Authors of this Abstract: K. Motivans, S. Apfelbaum Applied Ecological Services, Inc © THE NATURE CONSERVANCY 1815 North Lynn Street, Arlington, Virginia 22209 (703) 841 5300 The Nature Conservancy Element Stewardship Abstract Typha spp North American cattails I. IDENTIFIERS Scientific-Name: Typha spp. Common-Name: cattail Description: The cattail genus (Typha spp.) is an erect, perennial freshwater aquatic herb which can grow 3 or more meters in height. -

Typha Angustifolia and T
Final Report – Typha angustifolia and T. x glauca suppression at Mukwonago River Floodplain (TNC Lulu Lake Preserve) Walworth County, WI ∳Integrated Restorations, LLC Ecological Restoration & Land Management Services Integrating Ecological Theory and Research with Restoration Practices Final Report – Typha angustifolia and T. x glauca suppression at Mukwonago River Floodplain (TNC Lulu Lake Preserve) By Craig A. Annen Integrated Restorations, LLC June 2015 228 South Park Street Belleville, WI 53508 (608) 424-6997 (office) (608) 547-1713 (mobile) [email protected] Deliverable final report for contract number 60112-2012028 between The Nature Conservancy, Inc. and Integrated Restorations, LLC Final Report – Typha angustifolia suppression at Lulu Lake Preserve Page 2 Summary 1. A well-established population of Typha angustifolia was present in the Mukwonago River wetland and was found to be spreading at an accelerated rate. This expansion was of immediate management concern and an effective suppression method needed to be developed and empirically tested to protect the biological and ecological integrity of the wetland basin. 2. Both Typha angustifolia and T. x glauca were present in the Mukwonago River floodplain wetland. 3. T. angustifolia covered 4.83 acres of the wetland basin in 2010 (prior to management intervention), equal to 10.9% of the wetland area. T. x glauca occurred sporadically as individual culms throughout the wetland basin. 4. Cumulative species richness of the wetland basin (from 2012 through 2014) was S = 159, with a high proportion of conservative species. Cumulative Floristic Quality (FQI) was estimated at 67.2. 5. Three Wisconsin Special Concern species were detected during the three-year botanical survey: Solidago ohioensis (Ohio goldenrod), Triglochin maritima (bog arrow-grass), and Thelypteris simulata (bog fern). -

Fiber Cables in Leaf Blades of Typha Domingensis and Their Absence in Typha Elephantina: a Diagnostic Character for Phylogenetic Affinity
Israel Journal of Plant Sciences ISSN: 0792-9978 (Print) 2223-8980 (Online) Journal homepage: http://www.tandfonline.com/loi/tips20 Fiber cables in leaf blades of Typha domingensis and their absence in Typha elephantina: a diagnostic character for phylogenetic affinity Allan Witztum & Randy Wayne To cite this article: Allan Witztum & Randy Wayne (2016) Fiber cables in leaf blades of Typha domingensis and their absence in Typha elephantina: a diagnostic character for phylogenetic affinity, Israel Journal of Plant Sciences, 63:2, 116-123, DOI: 10.1080/07929978.2015.1096626 To link to this article: http://dx.doi.org/10.1080/07929978.2015.1096626 Published online: 12 Jan 2016. Submit your article to this journal Article views: 16 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tips20 Download by: [Weill Cornell Medical College] Date: 01 June 2016, At: 10:53 Israel Journal of Plant Sciences, 2016 Vol. 63, No. 2, 116À123, http://dx.doi.org/10.1080/07929978.2015.1096626 Fiber cables in leaf blades of Typha domingensis and their absence in Typha elephantina:a diagnostic character for phylogenetic affinity Allan Witztuma and Randy Wayneb* aDepartment of Life Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel; bLaboratory of Natural Philosophy, Section of Plant Biology, School of Integrative Plant Science, Cornell University, Ithaca, NY, 14853, USA (Received 10 August 2015; accepted 15 September 2015) Vertical fiber cables anchored in horizontal diaphragms traverse the air-filled lacunae of the tall, upright, spiraling leaf blades of Typha domingensis, T. -

Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management Sheel Bansal U.S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Plant Publications Health Inspection Service 6-21-2019 Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management Sheel Bansal U.S. Geological Survey Shane C. Lishawa Loyola University Chicago Sue Newman South Florida Water Management District Brian A. Tangen U.S. Geological Survey Douglas Wilcox FSUNolloYw Co thilleges aandt Br aocddkpoitriont al works at: https://digitalcommons.unl.edu/icwdm_usdanwrc See nePxat pratge of for the addiNtionatural aualthor Rsesources and Conservation Commons, Natural Resources Management and Policy Commons, Other Environmental Sciences Commons, Other Veterinary Medicine Commons, Population Biology Commons, Terrestrial and Aquatic Ecology Commons, Veterinary Infectious Diseases Commons, Veterinary Microbiology and Immunobiology Commons, Veterinary Preventive Medicine, Epidemiology, and Public Health Commons, and the Zoology Commons Bansal, Sheel; Lishawa, Shane C.; Newman, Sue; Tangen, Brian A.; Wilcox, Douglas; Albert, Dennis; Anteau, Michael J.; Chimney, Michael J.; Cressey, Ryann L.; DeKeyser, Edward; Elgersma, Kenneth J.; Finkelstein, Sarah A.; Freeland, Joanna; Grosshans, Richard; Klug, Page E.; Larkin, Daniel J.; Lawrence, Beth A.; Linz, George; Marburger, Joy; Noe, Gregory; Otto, Clint; Reo, Nicholas; Richards, Jennifer; Richardson, Curtis; Rodgers, LeRoy; Schrank, Amy J.; Svedarsky, Dan; Travis, Steven; Tuchman, Nancy; and Windham- Myers, Lisamarie, "Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management" (2019). USDA National Wildlife Research Center - Staff Publications. 2275. https://digitalcommons.unl.edu/icwdm_usdanwrc/2275 This Article is brought to you for free and open access by the U.S. -

Cattail Production Chain Development in Northeast Friesland
Cattail Production Chain Development in Northeast Friesland Cattail Cultivation Consultancy March. 2015 Academic Consultancy Training (YMC-60809) Period 3-4, 2015 Project 1482 Program Better Wetter Cattail Production Chain Development in Northeast Friesland Commissioner: Rianne Vos Knowledge Centre Northeast Friesland Cattail Cultivation Consultancy Manager: Lisanne van Beek Secretary: Denys van den Berg Controller: Wenyan Zheng Member: Xueyuan Leng, Maarten Verhoog, Egbert Bernard Wesselink II Summary In this report, we identified the whole production chain of cattail in Friesland. We selected three species, which are suitable for cultivation and production, from the approximately 30 species in the Typha genus. The growing conditions of T. latifolia, T. angustifolia and T. × glauca were investigated. Based on the cattail growing conditions and actual situation in Friesland, we investigated the natural locations and recommended several locations for cattail cultivation. Furthermore, we visited a growing location in Friesland and designed an artificial growing bed for a pilot project. Harvesting is also an essential section in this production chain. We studied and compared suitable harvesting methods in costs, advantages and disadvantages. Production is the section that makes cattail raw material into high-value products. We focused on bio-laminate and insulation material. The uses and production processes of these products were investigated. To identify the market we investigated the product price, market size, market demand and product advantages of bio-laminate and insulation material. We also compared cattail based products with traditional products in their current markets and made an assessment and give recommendations on cattail based production. Additionally, the farmers are essential stakeholders in this production chain. -

TYPHA REVIEW Ben Baldwin and Angie Cannon Utah State University November 2007
TYPHA REVIEW Ben Baldwin and Angie Cannon Utah State University November 2007 EXECUTIVE SUMMARY Typha spp., commonly referred to as “cattail,” is considered to be an invasive native in aquatic communities worldwide. Cattails’ tolerance to varying climatic conditions and environmental changes helps them achieve widespread dominance in a variety of habitats (Murkin and Ward 1980). Their tendency to spread rapidly and displace other species gives rise to several management concerns. Although Typha spp. is critical to maintaining habitat health, dense monospecific stands of Typha spp. reduce overall species diversity and richness. A 50:50 ratio of open water to emergent vegetation is a typical objective of wetland management. When cattail stands are equally interspersed with open water, they provide nest cover and building material for birds, food and shelter for muskrats and deer, and many other benefits (Beule 1979). Resource managers worldwide are taking steps to control the spread of Typha spp.. Understanding the characteristics, distribution, and impacts of Typha leads to better decision making and management planning. This document discusses the life history and characteristics of Typha, the impacts it may have on the environment, and the different management strategies that have been used to control it. Different species of Typha exist. Typha latifolia L. (broad-leaved cattail, common cattail) can be found in relatively undisturbed habitats, whereas Typha angustifolia L. (narrow- leaved cattail) and the hybrid, Typha x glauca, typically occur in more unstable and saline environments (Grace and Harrison 1986). Management strategies are relatively the same for the three different species. Several methods have been developed to effectively manage Typha spp. -

Supporting Documentation for Release Of
SUPPORTING DOCUMENTATION FOR RELEASE OF JACKSON-FRAZIER GERMPLASM MEADOW BARLEY (Hordeum brachyantherum) (source identified class pre-varietal release, natural track) compiled and written by Dale C. Darris (with sections of part D co-authored by Peter Gonzalves and Oregon State University researchers) (August 2008) A. Pre-Variety Name: Jackson-Frazier germplasm. Experimental Designations: PI-645564 and NRCS accession number 9056373. Species: Hordeum brachyantherum Nevski. Symbol: HOBR2. Synonyms include: Hordeum brachyantherum ssp. brachyantherum, Critesion brachyantherum, Critesion jubatum ssp. breviaristatum, Hordeum boreale, Hordeum jubatum ssp. breviaristatum, Hordeum jubatum var. boreale, Hordeum nodosum, Hordeum nodosum var. boreale. The common name is meadow barley. B. Origin and Breeding History of the Variety (Source Identified Class Germplasm): Jackson-Frazier Germplasm (9056373) meadow barley originates from a large population (several acres in size) growing in a wetland prairie within the Jackson- Frazier Wetland natural area in Benton County, Oregon: Sec 24 and 13, Township 11S, Range 5W, elevation 69 m (225 ft) above mean sea level. Coordinates: 123°14 min. 30 seconds west longitude, 44°37 min. north latitude. Mean annual precipitation: 107 cm (42 inches). Habitat: clay soil (Bashaw series), level exposure, wetland prairie plant community dominated by grass and grass-like herbaceous species including sedges (Carex spp.), tufted hairgrass (Deschampsia caespitosa), western sloughgrass (Beckmannia syzigachne), spiked bentgrass (Agrostis exarata), western mannagrass (Glyceria occidentalis) and cattail (Typha latifolia) . No information exists to suggest that this population was not naturally occurring at the time of initial and subsequent wild seed collections. Seed was originally collected In 1997 by Dale Darris then recollected in 1998 and 2000 by Dale Darris and in 2006 by Sonja Johnson. -
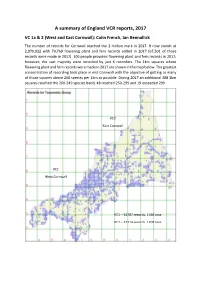
A Summary of England VCR Reports, 2017
A summary of England VCR reports, 2017 VC 1a & 2 (West and East Cornwall): Colin French, Ian Bennallick The number of records for Cornwall reached the 2 million mark in 2017. It now stands at 2,070,922 with 79,769 flowering plant and fern records added in 2017 (67,501 of those records were made in 2017). 106 people provided flowering plant and fern records in 2017; however, the vast majority were recorded by just 6 recorders. The 1km squares where flowering plant and fern records were made in 2017 are shown in the map below. The greatest concentration of recording took place in mid Cornwall with the objective of getting as many of those squares above 200 species per 1km as possible. During 2017 an additional 308 1km squares reached the 200-249 species band, 48 reached 250-299 and 19 exceeded 299. VC2 East Cornwall VC1 West Cornwall VC1 – 34787 records, 1166 taxa VC2 – 32714 records, 1209 taxa Number of species of flowering plants and ferns per 1km recorded since 1999 – up to 31st December 2017 Continued good progress was made in 2017 towards the ongoing monad survey which will result in the publication of the New Flora of Cornwall atlas. West Cornwall (VC1) is largely complete. 22 of the 3963 1km squares remain unvisited and of the 1909 1km squares that have less than 200 species recorded (see table below) it is estimated that 800-900 1km squares can be significantly improved. It should be noted that a large part of central VC2 consists of Bodmin Moor (circa 200 km2) where the maximum number of species per 1km square is close to 100 and many coastal squares only support low numbers of species.