Non-Native Typha Species and Hybrids Including: Typha Angustifolia L., Typha × Glauca Godr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moringa Oleifera 31.05.2005 8:55 Uhr Seite 1
Moringa oleifera 31.05.2005 8:55 Uhr Seite 1 Moringa oleifera III-4 Moringa oleifera LAM., 1785 syn.: Guilandina moringa LAM.; Hyperanthera moringa WILLD.; Moringa nux-ben PERR.; Moringa pterygosperma GAERTN., 1791 Meerrettichbaum, Pferderettichbaum Familie: Moringaceae Arabic: rawag Malayalam: murinna, sigru Assamese: saijna, sohjna Marathi: achajhada, shevgi Bengali: sajina Nepali: shobhanjan, sohijan Burmese: daintha, dandalonbin Oriya: sajina Chinese: la ken Portuguese: moringa, moringueiro English: drumstick tree, Punjabi: sainjna, soanjna horseradish tree, ben tree Sanskrit: shobhanjana, sigru French: moringe à graine ailée, Sinhalese: murunga morungue Spanish: ángela, ben, moringa Gujarati: midhosaragavo, saragavo Swahili: mrongo, mzunze Hindi: mungna, saijna, shajna Tamil: moringa, murungai Kannada: nugge Telegu: mulaga, munaga, Konkani: maissang, moring, tellamunaga moxing Urdu: sahajna Fig. 1: Flower detail (front and side view) Enzyklopädie der Holzgewächse – 40. Erg.Lfg. 6/05 1 Moringa oleifera 31.05.2005 8:55 Uhr Seite 2 Moringa oleifera III-4 Drumstick tree, also known as horseradish tree and ben It is cultivated and has become naturalized in other parts tree in English, is a small to medium-sized, evergreen or of Pakistan, India, and Nepal, as well as in Afghanistan, deciduous tree native to northern India, Pakistan and Bangladesh, Sri Lanka, Southeast Asia, West Asia, the Nepal. It is cultivated and has become naturalized well Arabian peninsula, East and West Africa, throughout the beyond its native range, including throughout South Asia, West Indies and southern Florida, in Central and South and in many countries of Southeast Asia, the Arabian Pe- America from Mexico to Peru, as well as in Brazil and ninsula, tropical Africa, Central America, the Caribbean Paraguay [17, 21, 29, 30, 51, 65]. -

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

(BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater
ISSN(Online): 2319-8753 ISSN (Print): 2347-6710 International Journal of Innovative Research in Science, Engineering and Technology (A High Impact Factor, Monthly, Peer Reviewed Journal) Visit: www.ijirset.com Vol. 8, Issue 1, January 2019 Evaluation of Bioaccumulation Factor (BAF), Bioconcentration Factor (BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater S. S. Shingadgaon1, B.L. Chavan2 Research Scholar, Department of Environmental Science, School of Earth Sciences, Solapur University, Solapur, MS, India1 Former Professor and Head, Department of Environmental Science, Solapur University Solapur and presently working at Department of Environmental Science, Dr.Babasaheb Ambedkar Marathwada University, Aurangabad, MS, India 2 ABSTRACT: Wastewaters receiving aquatic bodies are quiet complex in terms of pollutants, the transport and interactions with heavy metals. This complexity is primarily due to high variability of pollutants, contaminants and related parameters. The macrophytes are plausible bio-indicators of the pollution load and level of metals within the aquatic systems than the wastewater or sediment analyses. The potential ability of aquatic macrophytes in natural water bodies receiving municipal sewage from Solapur city was assessed. Data from the studies on macrophytes exposed to a mixed test bath of metals and examined to know their potentialities to accumulate heavy metals for judging their suitability for phytoremediation technology -

Asparagus
Give Your Family More of the Good Stuff! Asparagus Basics $ and $ n excellent sourc hop ave is a e of V gus ita < Look for stalks that are firm ra ps build stro m a hel ng bo in with tightly closed tips. Color sp ich ne K A wh s. , can be bright green, creamy white or even purple. < Stalks with the same thickness will cook in the same amount of time. < Fresh asparagus may be best Types of quality and lowest price when harvested locally, usually April Asparagus and May. Generally, thinner spears are < Asparagus is also available more delicate and tender; canned and frozen. thicker spears have stronger flavor and hearty texture. Asparagus Math: Thicker spears can be sliced on the diagonal into smaller One pound = 12 to 15 spears, pieces to cook more quickly. 9 to 10 inches long and 1/2 < Green – the most common to 3/4 inches thick type. = 3 cups trimmed < White – covered with soil as it grows to keep green 1 = 2 /2 cups cooked pigments from developing. Considered a delicacy and may cost more than green. tore Well < Purple – has more sugar and S less fiber than green. The skin aste Less is burgundy or purple but the W flesh is pale green to creamy I Refrigerate fresh asparagus for up white. Cooking may cause I to 5 days. Wash under cool running water more green to show. Less • Stand stalks in 1 inch of water just before using. Remove tough ends: commonly available and may like a flower bouquet and cover • Hold an end of the stalk in each cost more than green. -
(A) Journals with the Largest Number of Papers Reporting Estimates Of
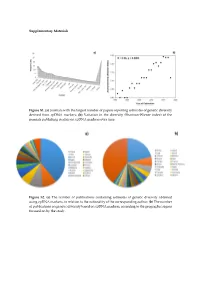
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Asparagus Densiflorus 'Sprengeri'
FPS051 Asparagus densiflorus ‘Sprengeri’ Sprengeri Asparagus Fern1 Edward F. Gilman, Ryan W. Klein, and Gail Hansen2 Introduction ‘Sprengeri’ Asparagus Fern is a rounded herbaceous perennial that is used in the landscape for its attractive, fine-textured foliage. This 1 to 4 foot-tall plant has true leaves that are scale-like and inconspicuous. The structures that most refer to as leaves are actually leaf-like branchlets called cladophylls. These tiny cladophylls are linear, flat- tened structures that are bright green in color. They occur singly or in groups of 3 or more at a node. The stems of this plant emerge directly from the ground and become woody and spiny, so be careful when handling this species. The thorns cause significant irritation to many people Figure 1. Full form—Asparagus densiflorus: ‘Sprengeri’ Sprengeri that handle the plant. Pretty, red, ovoid berries occur on asparagus fern. Asparagus densiflorus throughout the year. Several birds eat Credits: Edward F. Gilman, UF/IFAS and probably distribute the fruit. These fruits follow tiny, General Information white, flowers that occur in axillary racemes; the flowers are inconspicuous for the most part but fragrant. Seeds Scientific name: Asparagus densiflorus ‘Sprengeri’ germinate in the landscape and the plant has escaped into Pronunciation: ass-SPAR-uh-gus den-sif-FLOR-us natural habitats in parts of Florida. It can also become a Common name(s): ‘Sprengeri’ asparagus fern weed in your landscape. Family: Liliaceae Plant type: herbaceous; perennial USDA hardiness zones: 9B through 11 (Figure 2) Planting month for zone 7: year round Planting month for zone 8: year round Planting month for zone 9: year round Planting month for zone 10 and 11: year round Origin: not native to North America Invasive potential: potentially invasive 1. -

Fasciation. Lumina C
346 The Ohio Naturalist. [Vol. III, No. 3, FASCIATION. LUMINA C. RIDDLE. The phenomena of fasciation are sufficiently striking to attract the attention of the most casual observer, and the malformation occurs so frequently that nearly every person has seen one or more cases of it. It manifests itself usually by a remarkable broadening and flattening of the stem, crowded phyllotaxy and often spiral twisting and splitting of this broadened axis, although the portion of the plant affected and the exact character of the growth varies with the nature of the plant. Those having the rosette habit throughout their entire life, as the common dande- lion, show fasciation in the peduncle of the inflorescence. In the thistle (Fig. 2,) which has the rosette habit during the first year Fig. 1. a. Ailanthus glandulosus. b. Ranunculus abortivus, and is stemmed during the second year, it has only been observed in the second year's growth and affected the entire stalk. In the herbaceous hollow-stemmed plant of Ranunculus abortivus, {Fig. 1, b,) the entire stem was found fasciated and inside was found a reversed cylinder having the delicate epidermal layer within and a well developed ring of fibro-vascular tissue surround- ing it. In Erigeron philadelphicus the leaves were so closely Jan., 1903.] Fasciation. 347 compacted that the stem was entirely concealed while the top of the stalk was twisted down. In woody plants fasciated stems are nearly always split or twisted, often both, as shown in Ailanthus glandidosus {Fig. i, a.) Fasciation is found frequently occurring in man}- cultivated plants; the flowers, hyacinths, gladioli, narcissus, violets, gerani- u m s , nasturtiums ( Tropoeolum); the garden vegetables, cabbage or Brassica oleracea, and beets, Beta vulgaris ; and trees, Pinus, Thuya, Taxus, Salix, Alnus,Ulmus, Prunus and Populus. -

Proceedings of the Workshop on the Creation of Channels and Ponds Within Cattail Marshes on the Bay of Quinte, and a Conceptual Plan
PROCEEDINGS OF THE WORKSHOP ON THE CREATION OF CHANNELS AND PONDS WITHIN CATTAIL MARSHES ON THE BAY OF QUINTE, AND A CONCEPTUAL PLAN. PREPARED BY ANDY SMITH BAY OF QUINTE REMEDIAL ACTION PLAN JANUARY, 1995 PREFACE On August 17 and 18, 1994 a workshop was held to bring together scientists and members of , , the Bay of Quinte Implementation Advisory Committee (formally the Public Advisory Committee) to discuss enhancing Quinte wetlands by dredging channels and ponds in dense cattail stands. The goals for the workshop were to review the impacts of this technique, discuss its advantages and disadvantages, and design a new channel/pond system. This report is a summary of the workshop and a conceptual plan for a project based on recommendations from the workshop. If implemented this experimental/demonstration project will be studied to determine the effectiveness of creating open water areas within dense cattail stands for providing habitat for variety of species. TABLE OF CONTENTS 1.0 Workshop Introduction ...................................... 1 2.0 Summary of Workshop Presentations and Discussions:~,' ................. 3 2.1 A Literature Review of the Impacts to Wildlife of Channel Creation Through Monotypic Cattail Stands as Proposed at the Bay of Quinte Area of Concern 3 2.2 Studies Conducted on Wetlands in The Quinte Area, 1994 ........... 4 2.2.1 Review of Some Recent Wetland Enhancement Projects in the Quinte Area and Creation techniques. 4 2.2.2 Fisheries Assessment of Some Wetland Enhancement Projects in the Quinte Area . 5 2.2.3 List of Plants and Animals Observed During the Tour of Sawguin Creek Marsh, August 17, 1994 .......................... -

Studies of the Germination and Growth of Cattail in Relation to Marsh Management John William Bedish Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1964 Studies of the germination and growth of cattail in relation to marsh management John William Bedish Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Terrestrial and Aquatic Ecology Commons Recommended Citation Bedish, John William, "Studies of the germination and growth of cattail in relation to marsh management" (1964). Retrospective Theses and Dissertations. 16857. https://lib.dr.iastate.edu/rtd/16857 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. STUDIES OF THE GERMINATION AND GROWTH OF CATTAIL IN RELATION TO K~SH MANAGEMENT by John William Bedish A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of MASTER OF SCIENCE Major Subject: Wildlife Management Signatures have been redacted for privacy Iowa State University Of Science and Technology Ames, Iowa 1964 ii TABLE OF CONTENTS Page INTRODUCTION 1 LITERATURE REVIEW 3 Importance of Cattail to Marsh Animals 3 Relation of Water to Presence of Cattail 5 Seed Germination and Viability 8 METHODS OF STUDY 14 Greenhouse Studies 14 Field Studies 23 RESULTS 29 Greenhouse Studies 29 Field Studies 46 DISCUSSION 65 Effects of Moisture on Cattail 65 Management Recommendations 69 SUMMARY 76 LITERATURE CITED 79 ACKNOWLEDGEMENTS 84 1 INTRODUCTION The total area of wetlands available to waterfowl has been greatly reduced during the past century due mainly to drainage for agricultural purposes. -

Hybridization Dynamics of Invasive Cattail (Typhaceae) Stands at Pierce Cedar Creek Institute: a Molecular Analysis
HYBRIDIZATION DYNAMICS OF INVASIVE CATTAIL (TYPHACEAE) STANDS AT PIERCE CEDAR CREEK INSTITUTE: A MOLECULAR ANALYSIS Alex Graeff, Kelsey Huisman, and Dr. Pamela J. Laureto Department of Biological Sciences Grand Rapids Community College 143 Bostwick NE Grand Rapids, Michigan 49503 ABSTRACT Three cattail taxa are recognized in Michigan USA: native Typha latifolia (broad-leaf cattail), the invasive Typha angustifolia (narrow-leaf cattail), and the hybrid of these two species Typha × glauca. Typha angustifolia and T. × glauca are of special interest because of their ability to aggressively spread and out-compete the native cattail T. latifolia. Typha × glauca has been shown to out-compete both its parental taxa and produce monospecific stands. We surveyed the Pierce Cedar Creek Institute (PCCI) property for cattails and located 25 distinct cattail marshes. We determined the total area of cattail marsh at PCCI to be roughly 10% of the 267 ha property. Cattail individuals were sampled from each of the 25 stands and random amplified polymorphic DNA markers were used to identify the individuals to species. We found that 20 of the 25 stands were monospecific for the native cattail, T. latifolia. Five of the stands were mixtures of the native T. latifolia and the introduced T. angustifolia, and T. × glauca was found in two of the mixed stands. We recommend removal of the invasive T. angustifolia and T. × glauca individuals and the establishment of a monitoring plan in order to maintain the long-term health of the cattail marshes at PCCI. Keywords: Typha spp., RAPD markers, invasive species 1 INTRODUCTION Species of Typha L. (Typhaceae), commonly known as cattails, are highly productive emergent plants that grow in a variety of wetland habitats throughout the world (McManus et al. -

The Ayurvedic Pharmacopoeia of India
THE AYURVEDIC PHARMACOPOEIA OF INDIA PART- I VOLUME – V GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF AYUSH Contents | Monographs | Abbreviations | Appendices Legal Notices | General Notices Note: This e-Book contains Computer Database generated Monographs which are reproduced from official publication. The order of contents under the sections of Synonyms, Rasa, Guna, Virya, Vipaka, Karma, Formulations, Therapeutic uses may be shuffled, but the contents are same from the original source. However, in case of doubt, the user is advised to refer the official book. i CONTENTS Legal Notices General Notices MONOGRAPHS Page S.No Plant Name Botanical Name No. (as per book) 1 ËMRA HARIDRË (Rhizome) Curcuma amada Roxb. 1 2 ANISÍNA (Fruit) Pimpinella anisum Linn 3 3 A×KOLAH(Leaf) Alangium salviifolium (Linn.f.) Wang 5 4 ËRAGVËDHA(Stem bark) Cassia fistula Linn 8 5 ËSPHOÙË (Root) Vallaris Solanacea Kuntze 10 6 BASTËNTRÌ(Root) Argyreia nervosa (Burm.f.)Boj. 12 7 BHURJAH (Stem Bark) Betula utilis D.Don 14 8 CAÛÚË (Root) Angelica Archangelica Linn. 16 9 CORAKAH (Root Sock) Angelica glauca Edgw. 18 10 DARBHA (Root) Imperata cylindrica (Linn) Beauv. 21 11 DHANVAYËSAH (Whole Plant) Fagonia cretica Linn. 23 12 DRAVANTÌ(Seed) Jatropha glandulifera Roxb. 26 13 DUGDHIKË (Whole Plant) Euphorbia prostrata W.Ait 28 14 ELAVËLUKAê (Seed) Prunus avium Linn.f. 31 15 GAÛÚÌRA (Root) Coleus forskohlii Briq. 33 16 GAVEDHUKA (Root) Coix lachryma-jobi LInn 35 17 GHOÛÙË (Fruit) Ziziphus xylopyrus Willd. 37 18 GUNDRËH (Rhizome and Fruit) Typha australis -

Washington State Noxious Weeds
Changes to the 2014 Noxious Weed List Wendy DesCamp November 7, 2013 Today’s talk • Weed law review • New species for the 2014 noxious weed list • Other changes for 2014 to noxious weed list Noxious Weed • “Noxious weed” means a plant that when established is highly destructive, competitive, or difficult to control by cultural or chemical practices. RCW 17.10.10 The Noxious Weed Laws • RCW 17.10 – Limit economic loss due to the presence and spread of noxious weeds – Holds landowners responsible for controlling noxious weeds on their property – Noxious Weed Control Boards—county and state • RCW 17.04 and RCW 17.06 – Weed Districts The Noxious Weed Laws • WAC 16.750 – Weed list and schedule of penalties • WAC 16.752 – Prohibited plants, aka the quarantine list Noxious Weeds • Plants are noted as aggressive and highly difficult to control • Plants have a significant ecological impact, economic impact and/or cause harm to humans and other animals • 3 class of noxious weeds Class A Noxious Weeds • Class A consists of those noxious weeds – not native to the Washington – of limited distribution or are unrecorded in the state and – that pose a serious threat to the state • Eradication is required of all Class A noxious weeds • Currently 41 species Garlic mustard (Alliaria petiolata) Class B Noxious Weeds Scotch broom (Cytisus scoparius) • Class B: not native to the state and are of limited distribution or are unrecorded in a region of the state and that pose a serious threat to that region. • "Class B designate" means those Class B noxious weeds whose populations in a region or area are such that all seed production can be prevented within a calendar year.