Studies of the Germination and Growth of Cattail in Relation to Marsh Management John William Bedish Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater
ISSN(Online): 2319-8753 ISSN (Print): 2347-6710 International Journal of Innovative Research in Science, Engineering and Technology (A High Impact Factor, Monthly, Peer Reviewed Journal) Visit: www.ijirset.com Vol. 8, Issue 1, January 2019 Evaluation of Bioaccumulation Factor (BAF), Bioconcentration Factor (BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater S. S. Shingadgaon1, B.L. Chavan2 Research Scholar, Department of Environmental Science, School of Earth Sciences, Solapur University, Solapur, MS, India1 Former Professor and Head, Department of Environmental Science, Solapur University Solapur and presently working at Department of Environmental Science, Dr.Babasaheb Ambedkar Marathwada University, Aurangabad, MS, India 2 ABSTRACT: Wastewaters receiving aquatic bodies are quiet complex in terms of pollutants, the transport and interactions with heavy metals. This complexity is primarily due to high variability of pollutants, contaminants and related parameters. The macrophytes are plausible bio-indicators of the pollution load and level of metals within the aquatic systems than the wastewater or sediment analyses. The potential ability of aquatic macrophytes in natural water bodies receiving municipal sewage from Solapur city was assessed. Data from the studies on macrophytes exposed to a mixed test bath of metals and examined to know their potentialities to accumulate heavy metals for judging their suitability for phytoremediation technology -
(A) Journals with the Largest Number of Papers Reporting Estimates Of
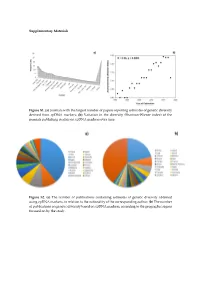
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Proceedings of the Workshop on the Creation of Channels and Ponds Within Cattail Marshes on the Bay of Quinte, and a Conceptual Plan
PROCEEDINGS OF THE WORKSHOP ON THE CREATION OF CHANNELS AND PONDS WITHIN CATTAIL MARSHES ON THE BAY OF QUINTE, AND A CONCEPTUAL PLAN. PREPARED BY ANDY SMITH BAY OF QUINTE REMEDIAL ACTION PLAN JANUARY, 1995 PREFACE On August 17 and 18, 1994 a workshop was held to bring together scientists and members of , , the Bay of Quinte Implementation Advisory Committee (formally the Public Advisory Committee) to discuss enhancing Quinte wetlands by dredging channels and ponds in dense cattail stands. The goals for the workshop were to review the impacts of this technique, discuss its advantages and disadvantages, and design a new channel/pond system. This report is a summary of the workshop and a conceptual plan for a project based on recommendations from the workshop. If implemented this experimental/demonstration project will be studied to determine the effectiveness of creating open water areas within dense cattail stands for providing habitat for variety of species. TABLE OF CONTENTS 1.0 Workshop Introduction ...................................... 1 2.0 Summary of Workshop Presentations and Discussions:~,' ................. 3 2.1 A Literature Review of the Impacts to Wildlife of Channel Creation Through Monotypic Cattail Stands as Proposed at the Bay of Quinte Area of Concern 3 2.2 Studies Conducted on Wetlands in The Quinte Area, 1994 ........... 4 2.2.1 Review of Some Recent Wetland Enhancement Projects in the Quinte Area and Creation techniques. 4 2.2.2 Fisheries Assessment of Some Wetland Enhancement Projects in the Quinte Area . 5 2.2.3 List of Plants and Animals Observed During the Tour of Sawguin Creek Marsh, August 17, 1994 .......................... -

Hybridization Dynamics of Invasive Cattail (Typhaceae) Stands at Pierce Cedar Creek Institute: a Molecular Analysis
HYBRIDIZATION DYNAMICS OF INVASIVE CATTAIL (TYPHACEAE) STANDS AT PIERCE CEDAR CREEK INSTITUTE: A MOLECULAR ANALYSIS Alex Graeff, Kelsey Huisman, and Dr. Pamela J. Laureto Department of Biological Sciences Grand Rapids Community College 143 Bostwick NE Grand Rapids, Michigan 49503 ABSTRACT Three cattail taxa are recognized in Michigan USA: native Typha latifolia (broad-leaf cattail), the invasive Typha angustifolia (narrow-leaf cattail), and the hybrid of these two species Typha × glauca. Typha angustifolia and T. × glauca are of special interest because of their ability to aggressively spread and out-compete the native cattail T. latifolia. Typha × glauca has been shown to out-compete both its parental taxa and produce monospecific stands. We surveyed the Pierce Cedar Creek Institute (PCCI) property for cattails and located 25 distinct cattail marshes. We determined the total area of cattail marsh at PCCI to be roughly 10% of the 267 ha property. Cattail individuals were sampled from each of the 25 stands and random amplified polymorphic DNA markers were used to identify the individuals to species. We found that 20 of the 25 stands were monospecific for the native cattail, T. latifolia. Five of the stands were mixtures of the native T. latifolia and the introduced T. angustifolia, and T. × glauca was found in two of the mixed stands. We recommend removal of the invasive T. angustifolia and T. × glauca individuals and the establishment of a monitoring plan in order to maintain the long-term health of the cattail marshes at PCCI. Keywords: Typha spp., RAPD markers, invasive species 1 INTRODUCTION Species of Typha L. (Typhaceae), commonly known as cattails, are highly productive emergent plants that grow in a variety of wetland habitats throughout the world (McManus et al. -

Cattail, Typha Latifolia
BROAD-LEAVED the young shoots are steamed they taste like cabbage. The base of the stem where it attaches to the rhizome CATTAIL can be boiled or roasted like potatoes. The young flower stalks can be taken out of their sheaths and Typha latifolia L. can be boiled or steamed just like corn (Roos-Collins plant symbol = TYLA 1990; Clarke 1977). Contributed By: USDA, NRCS, National Plant Data Cattail pollen is a fine substitute for flours. It is a Center & Idaho Plant Materials Center bright yellow or green color, and turns pancakes, cookies or biscuits a pretty yellow color (which Alternate Names children love). The rhizomes (underground stems) Flags, rushes, bulrushes, cat o’nine tails, Cossack and lower stems have a sweet flavor and can be eaten raw, baked, roasted, or broiled. Cattail rhizomes are fairly high in starch content; this is usually listed at about 30% to 46%. The core can be ground into flour. One acre of cattails would yield about 6,475 pounds of flour (Harrington 1972). This flour would probably contain about 80 % carbohydrates and around 6% to 8% protein. Since cattail occurs around the world, it is a potential source of food for the worlds' population. Newly emerging shoots of cattails are edible, with delicate flavor and crispy asparagus like texture (Glenn Keator, Linda Yamane, Ann Lewis 1995). The end of a new stem of cattail is popular for eating with Washoes (Murphy 1959). When mixed with tallow, the brown fuzz can be chewed like gum. The Klamath and Modocs of northern California and southern Oregon make flexible baskets of twined tule or cattail. -

ABSTRACT Title of Dissertation: SECRETIVE MARSHBIRDS of URBAN WETLANDS in the WASHINGTON, DC METROPOLITAN AREA Patrice Nielson
ABSTRACT Title of Dissertation: SECRETIVE MARSHBIRDS OF URBAN WETLANDS IN THE WASHINGTON, DC METROPOLITAN AREA Patrice Nielson, Doctor of Philosophy 2016 Dissertation directed by: Dr. William Bowerman and Dr. Andrew Baldwin Environmental Science and Technology Secretive marshbirds are in decline across their range and are species of greatest conservation need in state Wildlife Action Plans. However, their secretive nature means there is relatively sparse information available on their ecology. There is demand for this information in the Washington, DC area for updating conservation plans and guiding wetland restoration. Rapid Wetland Assessment Methods are often used to monitor success of restoration but it is unknown how well they indicate marshbird habitat. Using the Standardized North American Marshbird Monitoring Protocol, I surveyed 51 points in 25 marshes in the DC area in 2013 – 2015. I also collected data on marsh area, buffer width, vegetation/water interspersion, vegetation characteristics, flooding, and invertebrates. At each bird survey point I assessed wetland quality using the Floristic Quality Assessment Index (FQAI) and California Rapid Wetland Assessment (CRAM) methods. I used Program Presence to model detection and occupancy probabilities of secretive marshbirds as a function of habitat variables. I found king rails (Rallus elegans) at five survey sites and least bittern (Ixobrychus exilis) at thirteen survey sites. Secretive marshbirds were using both restored and natural marshes, marshes with and without invasive plant species, and marshes with a variety of dominant vegetation species. King rail occupancy was positively correlated with plant diversity and invertebrate abundance and weakly negatively correlated with persistent vegetation. Least bittern occupancy was strongly negatively correlated woody vegetation and invertebrate abundance and weakly positively correlated with persistent vegetation. -

Narrowleaf Cattail
NARROWLEAF The Klamath and Modoc peoples of northern California and southern Oregon made flexible CATTAIL baskets of twined cattail. Cattails were also twined to form mats of varying sizes for sleeping, sitting, Typha angustifolia L. working, entertaining, covering doorways, providing plant symbol = TYAN shade, and a myriad of other uses. Lengths of cattail were plied into rope or other size cordage, and cattail Contributed By: USDA, NRCS, National Plant Data rope was used in some areas to bind bundles of tule Center & Idaho Plant Materials Center into tule boats. Air pockets or aerenchyma in the stems provided the buoyancy for good boat-building Alternate Names material. flags, rushes, bulrushes, cat o’nine The Cahuilla Indians used the stalks for matting, tails, Cossack bedding material, and ceremonial bundles (Barrows asparagus, reed 1967). Some tribes used the leaves and sheath bases mace, baco as caulking materials. Apaches used the pollen in female puberty ceremonies. After dipping the spike Uses in coal oil, the stalk makes a fine torch. The fluff can Caution: This also be used as tinder, insulation, or for lining baby species can be very cradleboards. The down is used for baby beds invasive in disturbed (Murphey 1959). wetlands. Please read about the Wildlife: The multitudes of tiny, wind-carried seeds environmental are too small and too hairy to be attractive to birds concerns under (Hotchkiss and Dozier 1949). In a few exceptions, Management. the seeds are eaten by several duck species. Cattail rootstocks are much more valuable as food for Ethnobotanic: All wildlife than are the seeds. Geese and muskrats parts of the cattail prefer the stems and roots. -

ELEMENT STEWARDSHIP ABSTRACT for Typha Spp. North
ELEMENT STEWARDSHIP ABSTRACT for Typha spp. North American Cattails To the User: Element Stewardship Abstracts (ESAs) are prepared to provide The Nature Conservancy's Stewardship staff and other land managers with current management-related information on those species and communities that are most important to protect, or most important to control. The abstracts organize and summarize data from numerous sources including literature and researchers and managers actively working with the species or community. We hope, by providing this abstract free of charge, to encourage users to contribute their information to the abstract. This sharing of information will benefit all land managers by ensuring the availability of an abstract that contains up-to-date information on management techniques and knowledgeable contacts. Contributors of information will be acknowledged within the abstract and receive updated editions. To contribute information, contact the editor whose address is listed at the end of the document. For ease of update and retrievability, the abstracts are stored on computer at the national office of The Nature Conservancy. This abstract is a compilation of available information and is not an endorsement of particular practices or products. Please do not remove this cover statement from the attached abstract. Authors of this Abstract: K. Motivans, S. Apfelbaum Applied Ecological Services, Inc © THE NATURE CONSERVANCY 1815 North Lynn Street, Arlington, Virginia 22209 (703) 841 5300 The Nature Conservancy Element Stewardship Abstract Typha spp North American cattails I. IDENTIFIERS Scientific-Name: Typha spp. Common-Name: cattail Description: The cattail genus (Typha spp.) is an erect, perennial freshwater aquatic herb which can grow 3 or more meters in height. -

Lake Sawyer Integrated Aquatic Vegetation Management Plan King County, Washington
Lake Sawyer Integrated Aquatic Vegetation Management Plan King County, Washington February 2015 Alternate Formats Available Lake Sawyer Integrated Aquatic Vegetation Management Plan King County, Washington Prepared for: The City of Black Diamond Submitted by: King County Water and Land Resources Division Department of Natural Resources and Parks Funded in part by: The Washington State Department of Ecology Lake Sawyer Integrated Aquatic Vegetation Management Plan Acknowledgements The City of Black Diamond and King County Department of Natural Resources and Parks’ Water and Land Resources Division would like to thank the members of the Steering Committee for their help in the development of the lake Sawyer Integrated Aquatic Vegetation Management Plan. Additionally we would like to thank Jennifer Parsons and Lizbeth Seebacher from the Washington State Department of Ecology for their review and expertise in aquatic noxious weeds. Citation King County. 2015 Lake Sawyer Integrated Aquatic Vegetation Management Plan. Prepared by King County Freshwater Assessment Unit, Water and Land Resources Division. Seattle, Washington. King County Science and Technical Support Section i February 2015 Lake Sawyer Integrated Aquatic Vegetation Management Plan Table of Contents Executive Summary............................................................................................................................................. vi 1.0. Introduction ............................................................................................................................................. -

Typha × Glauca Godr., 香蒲属(香蒲科) 中国新记录杂种及其形态特征
第37卷 第1期 水生生物学报 Vol. 37, No.1 2013 年 1 月 ACTA HYDROBIOLOGICA SINICA Jan., 2 0 1 3 doi: 10.7541/2013.29 Typha × glauca Godr., 香蒲属(香蒲科) 中国新记录杂种及其形态特征 朱秀玉 王 东 (华中师范大学生命科学学院, 武汉 430079) 摘要: 香蒲属(Typha L.)为多年生水生或沼生草本植物, 种间存在十分普遍的杂交现象, 其中一些杂种在湿 地生态系统中有重要的作用。在查阅大量腊叶标本基础上, 结合野外居群生物学工作, 作者发现中国一新记 录杂种, 即 T. × glauca Godr. (T. angustifolia L. × T. latifolia L.), 并新拟“粉绿香蒲”作该杂种的中文名。对粉 绿香蒲的形态特征进行了研究, 讨论了其与亲本水烛和宽叶香蒲的区别, 并给出检索表。 关键词: 香蒲属; 水烛; 宽叶香蒲; 粉绿香蒲; 新记录 中图分类号: Q949.7 文献标识码: A 文章编号: 1000-3207(2013)01-0029-05 香蒲属(Typha L.)隶属香蒲科(Typhaceae), 世界 中国科学院成都生物研究所植物标本馆(CDBI)、中 约 24 种[1—4], 分布于南北两半球的温带和热带地 国科学院武汉植物园植物标本馆(HIB)、武汉大学植 区。我国约有 12 种, 南北广泛分布[5, 6]。该属种间 物标本馆(WH)、四川大学植物标本馆(SZ)等所有馆 存在十分普遍的杂交现象, 欧洲、美洲等地已经报 藏香蒲标本以及我们野外采集的香蒲标本[存放华 道有 7 个杂种[7—13], 其中 T. angustifolia × T. latifolia 中师范大学植物标本馆(CCNU)], 尤其是馆藏标本 (= T. × glauca Godr.)、T. angustifolia × T. domingensis 中被鉴定为水烛(T. angustifolia L.)和宽叶香蒲(T. 和 T. domingensis × T. latifolia (= T. × provincialis latifolia L.)的标本。形态特征对比研究包括外部形 Camus)[8]等 3 个杂种经过细胞遗传学实验确认。在 态、花、果实、种子及花粉等。 欧洲和北美等地, 香蒲属杂种是湿地尤其是在严重 1.2 形态观察和测量 人为干扰或易受较大水位波动影响生境中的优势植 用直接观察法研究植物的营养器官和繁殖器官 物, 它们在湿地生态系统中的作用一直受到植物学 的形态特征。在 NIKON SMZ-1500 体视显微镜下观 家和生态学家的关注[9—11, 14—19]。 察花、果实和种子, 在 OLYMPUS BX51 型光学显微 我们在研究香蒲属标本过程中, 发现采自新疆 镜下观察花粉粒, 并分别测量大小和拍照。取腊叶 西部的 10 份标本, 其形态特征属于杂种 T. × glauca 标本的花药, 并经冰醋酸浸泡、醋酸酐处理、离心 Godr.的范畴。该杂种以前在国内未见报道, 属于中 沉淀淘洗后制作装片, 用于花粉粒的观察和大小测 国的一分布新记录, 本文予以报道。 量。选取 10 个以上样本用于各项数据在显微镜下性 状的测量, 求平均值。 1 材料与方法 2 分类与形态学描述 1.1 腊叶标本研究 研究存放中国科学院植物研究所植物标本馆 粉绿香蒲(杂种) 新拟 图版Ⅰ: 6—11, 20 (PE)、中国科学院华南植物园植物标本馆(IBSC)、 Typha × glauca Godr. (T. angustifolia L. ×T. 收稿日期: 2011-12-06; 修订日期: 2012-04-13 基金项目: 国家自然科学基金项目(30870151); 中国科学院植物研究所系统与进化植物学国家重点实验室开放课题(LSEB2011- 06); 国家自然科学基金委重大国际合作项目(31110103911); 中国科学院战略性先导科技专项—应对气候变化的碳收 支认证及相关问题(XDA05050206)项目资助 作者简介: 朱秀玉(1984—), 女, 山东临沂人; 硕士研究生; 主要从事植物分类学和生态学研究。E-mail: [email protected] 通信作者: 王东, E-mail: [email protected] 30 水生生物学报 37 卷 latifolia L.). -

Floristic Quality and Wildlife Habitat Assessment of Playas in Eastern Colorado
Floristic Quality and Wildlife Habitat Assessment of Playas in Eastern Colorado Final Report to the United States Environmental Protection Agency and the Colorado Division of Wildlife April 2009 Alison Banks Cariveau, Research Division Director David Pavlacky, Spatial Ecologist Rocky Mountain Bird Observatory P.O. Box 1232 14500 Lark Bunting Lane Brighton, CO 80603 303.659.4348 www.rmbo.org Floristic Quality and Wildlife Habitat Assessment of Playas in Eastern Colorado Acknowledgements Final Report to the US EPA and CDOW ROCKY MOUNTAIN BIRD OBSERVATORY Mission: To conserve birds and their habitats Vision: Native bird populations sustained in healthy ecosystems Core Values: (Our goals for achieving our mission) 1. Science provides the foundation for effective bird conservation. 2. Education is critical to the success of bird conservation. 3. Stewardship of birds and their habitats is a shared responsibility. RMBO accomplishes its mission by: Partnering with state and federal natural resource agencies, private landowners, schools, and other nonprofits for conservation. Studying bird responses to habitat conditions, ecological processes, and management actions to provide scientific information that guides bird conservation efforts. Monitoring long-term trends in bird populations for our region. Providing active, experiential, education programs that create an awareness and appreciation for birds. Sharing the latest information in land management and bird conservation practices. Developing voluntary, working partnerships with landowners to engage them in conservation. Working across political and jurisdictional boundaries including counties, states, regions, and national boundaries. Our conservation work emphasizes the Western United States including the Great Plains, as well as Latin America. Creating informed publics and building consensus for bird conservation needs. -

Nuclear Genes, Matk and the Phylogeny of the Poales
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Nuclear genes, matK and the phylogeny of the Poales Hochbach, Anne ; Linder, H Peter ; Röser, Martin Abstract: Phylogenetic relationships within the monocot order Poales have been well studied, but sev- eral unrelated questions remain. These include the relationships among the basal families in the order, family delimitations within the restiid clade, and the search for nuclear single-copy gene loci to test the relationships based on chloroplast loci. To this end two nuclear loci (PhyB, Topo6) were explored both at the ordinal level, and within the Bromeliaceae and the restiid clade. First, a plastid reference tree was inferred based on matK, using 140 taxa covering all APG IV families of Poales, and analyzed using parsimony, maximum likelihood and Bayesian methods. The trees inferred from matK closely approach the published phylogeny based on whole-plastome sequencing. Of the two nuclear loci, Topo6 supported a congruent, but much less resolved phylogeny. By contrast, PhyB indicated different phylo- genetic relationships, with, inter alia, Mayacaceae and Typhaceae sister to Poaceae, and Flagellariaceae in a basally branching position within the Poales. Within the restiid clade the differences between the three markers appear less serious. The Anarthria clade is first diverging in all analyses, followed by Restionoideae, Sporadanthoideae, Centrolepidoideae and Leptocarpoideae in the matK and Topo6 data, but in the PhyB data Centrolepidoideae diverges next, followed by a paraphyletic Restionoideae with a clade consisting of the monophyletic Sporadanthoideae and Leptocarpoideae nested within them. The Bromeliaceae phylogeny obtained from Topo6 is insufficiently sampled to make reliable statements, but indicates a good starting point for further investigations.