ABSTRACT Title of Dissertation: SECRETIVE MARSHBIRDS of URBAN WETLANDS in the WASHINGTON, DC METROPOLITAN AREA Patrice Nielson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

(BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater
ISSN(Online): 2319-8753 ISSN (Print): 2347-6710 International Journal of Innovative Research in Science, Engineering and Technology (A High Impact Factor, Monthly, Peer Reviewed Journal) Visit: www.ijirset.com Vol. 8, Issue 1, January 2019 Evaluation of Bioaccumulation Factor (BAF), Bioconcentration Factor (BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater S. S. Shingadgaon1, B.L. Chavan2 Research Scholar, Department of Environmental Science, School of Earth Sciences, Solapur University, Solapur, MS, India1 Former Professor and Head, Department of Environmental Science, Solapur University Solapur and presently working at Department of Environmental Science, Dr.Babasaheb Ambedkar Marathwada University, Aurangabad, MS, India 2 ABSTRACT: Wastewaters receiving aquatic bodies are quiet complex in terms of pollutants, the transport and interactions with heavy metals. This complexity is primarily due to high variability of pollutants, contaminants and related parameters. The macrophytes are plausible bio-indicators of the pollution load and level of metals within the aquatic systems than the wastewater or sediment analyses. The potential ability of aquatic macrophytes in natural water bodies receiving municipal sewage from Solapur city was assessed. Data from the studies on macrophytes exposed to a mixed test bath of metals and examined to know their potentialities to accumulate heavy metals for judging their suitability for phytoremediation technology -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Bird List Column A: We Should Encounter (At Least a 90% Chance) Column B: May Encounter (About a 50%-90% Chance) Column C: Possible, but Unlikely (20% – 50% Chance)
THE PHILIPPINES Prospective Bird List Column A: we should encounter (at least a 90% chance) Column B: may encounter (about a 50%-90% chance) Column C: possible, but unlikely (20% – 50% chance) A B C Philippine Megapode (Tabon Scrubfowl) X Megapodius cumingii King Quail X Coturnix chinensis Red Junglefowl X Gallus gallus Palawan Peacock-Pheasant X Polyplectron emphanum Wandering Whistling Duck X Dendrocygna arcuata Eastern Spot-billed Duck X Anas zonorhyncha Philippine Duck X Anas luzonica Garganey X Anas querquedula Little Egret X Egretta garzetta Chinese Egret X Egretta eulophotes Eastern Reef Egret X Egretta sacra Grey Heron X Ardea cinerea Great-billed Heron X Ardea sumatrana Purple Heron X Ardea purpurea Great Egret X Ardea alba Intermediate Egret X Ardea intermedia Cattle Egret X Ardea ibis Javan Pond-Heron X Ardeola speciosa Striated Heron X Butorides striatus Yellow Bittern X Ixobrychus sinensis Von Schrenck's Bittern X Ixobrychus eurhythmus Cinnamon Bittern X Ixobrychus cinnamomeus Black Bittern X Ixobrychus flavicollis Black-crowned Night-Heron X Nycticorax nycticorax Western Osprey X Pandion haliaetus Oriental Honey-Buzzard X Pernis ptilorhynchus Barred Honey-Buzzard X Pernis celebensis Black-winged Kite X Elanus caeruleus Brahminy Kite X Haliastur indus White-bellied Sea-Eagle X Haliaeetus leucogaster Grey-headed Fish-Eagle X Ichthyophaga ichthyaetus ________________________________________________________________________________________________________ WINGS ● 1643 N. Alvernon Way Ste. 109 ● Tucson ● AZ ● 85712 ● www.wingsbirds.com -
(A) Journals with the Largest Number of Papers Reporting Estimates Of
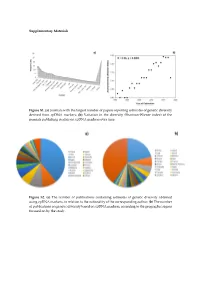
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Notas De La Anidación Del Rascón De Manglar Rallus Longirostris (Gruiformes: Rallidae) En El Salvador
Revista Multidisciplinaria de la Universidad de El Salvador • Revista Minerva (2020) 3(1) • pp. 141-150 Plataforma digital de la revista: https://minerva.sic.ues.edu.sv Notas de la anidación del Rascón de Manglar Rallus longirostris (Gruiformes: Rallidae) en El Salvador Nesting notes of the Mangrove Rail Rallus longirostris (Gruiformes: Rallidae) in El Salvador Luis Pineda1, Larissa Beltrán2, Moisés Herrera3, Alcides Sorto3 RESUMEN Presentamos información de la anidación del Rascón de Manglar Rallus longirostris en Bahía de La Unión, que representa la primera zona reproductiva de esta especie en El Salvador. Describimos características de los nidos, huevos, polluelos y su ubicación. La especie fue registrada por primera vez en 2013 mediante la grabación de vocalizaciones en el Golfo de Fonseca. El nido se encontró a una altura de 1 m, elaborado de ramas de mangle entrelazadas y una base de hojas, en forma de canasta de 28 cm de diámetro, contenía cinco huevos de coloración blanquecinos con manchas marrones, los cuales median 4.5x3.5 cm, el período reproductivo es de mayo a noviembre. Palabras clave: Anidación, La Unión, Rascón de Manglar, Rallus longirostris, reproducción. ABSTRACT We present information on the nesting of the Mangrove Rail Rallus longirostris in Bahía de La Unión, which represents the first reproductive area of this species in El Salvador. We describe the characteristics of nests, eggs, chicks and their location. The species was first recorded in 2013 when recording vocalizations in the Golfo de Fonseca. The nest was found at a height of 1 m, made of interlocking mangrove branches and a base of leaves, in the form of a 28 cm diameter basket, containing five whitish eggs with brown spots, measuring 4.5x3.5 cm, the reproductive period is from May to November. -

Aquatic Vascular Plant Species Distribution Maps
Appendix 11.5.1: Aquatic Vascular Plant Species Distribution Maps These distribution maps are for 116 aquatic vascular macrophyte species (Table 1). Aquatic designation follows habitat descriptions in Haines and Vining (1998), and includes submergent, floating and some emergent species. See Appendix 11.4 for list of species. Also included in Appendix 11.4 is the number of HUC-10 watersheds from which each taxon has been recorded, and the county-level distributions. Data are from nine sources, as compiled in the MABP database (plus a few additional records derived from ancilliary information contained in reports from two fisheries surveys in the Upper St. John basin organized by The Nature Conservancy). With the exception of the University of Maine herbarium records, most locations represent point samples (coordinates were provided in data sources or derived by MABP from site descriptions in data sources). The herbarium data are identified only to township. In the species distribution maps, town-level records are indicated by center-points (centroids). Figure 1 on this page shows as polygons the towns where taxon records are identified only at the town level. Data Sources: MABP ID MABP DataSet Name Provider 7 Rare taxa from MNAP lake plant surveys D. Cameron, MNAP 8 Lake plant surveys D. Cameron, MNAP 35 Acadia National Park plant survey C. Greene et al. 63 Lake plant surveys A. Dieffenbacher-Krall 71 Natural Heritage Database (rare plants) MNAP 91 University of Maine herbarium database C. Campbell 183 Natural Heritage Database (delisted species) MNAP 194 Rapid bioassessment surveys D. Cameron, MNAP 207 Invasive aquatic plant records MDEP Maps are in alphabetical order by species name. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

REGUA Bird List July 2020.Xlsx
Birds of REGUA/Aves da REGUA Updated July 2020. The taxonomy and nomenclature follows the Comitê Brasileiro de Registros Ornitológicos (CBRO), Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee, updated June 2015 - based on the checklist of the South American Classification Committee (SACC). Atualizado julho de 2020. A taxonomia e nomenclatura seguem o Comitê Brasileiro de Registros Ornitológicos (CBRO), Lista anotada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos, atualizada em junho de 2015 - fundamentada na lista do Comitê de Classificação da América do Sul (SACC). -

La Mancha, Coto Donana & Extremadura 2017
Field Guides Tour Report Spain: La Mancha, Coto Donana & Extremadura 2017 May 6, 2017 to May 18, 2017 Chris Benesh & Godfried Schreur For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Spectacular skies greeted us during our visit to old Trujillo in the heart of Extremadura. Photo by guide Chris Benesh. So many birds around that you don´t know which to choose and observe. Do you recognize this feeling? We experienced many of these exciting moments in Spain during the Field Guides tour in May. It started straight away, on the first day, overlooking the natural lagoons of La Mancha Húmeda, where we had the chance to observe a great variety of species of ducks, grebes, terns, and passerines. The highlights here were the White-headed Duck, Eared Grebe, Red-crested Pochard, Whiskered Tern and Penduline Tit. In the National Park of Coto Donana again we found ourselves surrounded by birds: larks, bee-eaters, flamingos, Great Reed Warblers, Glossy Ibis, Squacco and Purple herons and a surprisingly well showing Little Bittern. With a bit of searching, scanning and listening we were able to also detect Red-knobbed Coot, Marbled Teal and Isabelline (Western Olivaceous) Warbler. Later in the week, close to Trujillo (Extremadura), we all enjoyed the excursion on the open, rolling plains, with Great and Little bustards, Eurasian Roller, Hoopoe, Calandra Lark, Montagu´s Harrier and many, many White Storks. For the shy Black Storks we had to wait one day more. In Monfrague National Park we discovered 3 pairs nesting on the breathtaking cliff of Peña Falcón. -

Ixobrychus Exilis (Gmelin) Leastleast Bitternbittern, Page 1
Ixobrychus exilis (Gmelin) Leastleast Bitternbittern, Page 1 State Distribution Best Survey Period Copyright The Otter Side Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Status: State threatened state.” Wood (1951) identified the species as a summer resident and common in southern tiers of counties and Global and state rank: G5/S2 Cheboygan County, but rare and local in the Upper Peninsula. Least bittern was later described by Payne Family: Ardeidae – Herons, Egrets, and Bitterns (1983) as an uncommon transient and summer resident, with nesting confirmed in 27 counties. Michigan Total range: Five subspecies of least bittern are found Breeding Bird Atlas (Atlas) surveys conducted in the throughout much of North, Central, and South America 1980s confirmed breeding in 20 survey blocks in 17 (Gibbs et al. 1992). In North America, this species is counties (Adams 1991). All of these observations primarily restricted to the eastern U.S., ranging from occurred in the Lower Peninsula, with the number of the Great Plains states eastward to the Atlantic Coast blocks and counties with confirmed breeding nearly split and north to the Great Lakes region and the New between the northern (9 blocks in 8 counties) and England states (Evers 1994). Western populations are southern (11 blocks in 9 counties) Lower Peninsula concentrated in low-lying areas of the Central Valley (Adams 1991). Researchers confirmed nesting at and Modoc Plateau of California, the Klamath and several sites on Saginaw Bay and observed possible Malheur basins of Oregon, and along the Colorado breeding in Munuscong Bay wetlands (Chippewa River in southwest Arizona and southeast California County) during avian studies conducted in the mid- (Gibbs et al. -

Missouriensis Volume 28 / 29
Missouriensis Volume 28/29 (2008) In this issue: Improved Status of Auriculate False Foxglove (Agalinis auriculata) in Missouri in 2007 Tim E. Smith, Tom Nagel, and Bruce Schuette ......................... 1 Current Status of Yellow False Mallow (Malvastrum hispidum) in Missouri Tim E. Smith.................................................................................... 5 Heliotropium europaeum (Heliotropiaceae) New to Missouri Jay A. Raveill and George Yatskievych ..................................... 10 Melica mutica (Poaceae) New for the Flora of Missouri Alan E. Brant ................................................................................. 18 Schoenoplectus californicus (Cyperaceae) New to Missouri Timothy E. Vogt and Paul M. McKenzie ................................. 22 Flora of Galloway Creek Nature Park, Howell County, Missouri Bill Summers .................................................................................. 27 Journal of the Missouri Native Plant Society Missouriensis, Volume 28/29 2008 1 IMPROVED STATUS OF AURICULATE FALSE FOXGLOVE (AGALINIS AURICULATA) IN MISSOURI IN 2007 Tim E. Smith Missouri Department of Conservation P.O. Box 180, Jefferson City, MO 65102-0180 Tom Nagel Missouri Department of Conservation 701 James McCarthy Drive St. Joseph, MO 64507-2194 Bruce Schuette Missouri Department of Natural Resources Cuivre River State Park 678 State Rt. 147 Troy, MO 63379 Populations of annual plant species are known to have periodic “boom” and “bust” years as well as years when plant numbers more closely approach long-term averages. In tracking populations of plant species of conservation concern (Missouri Natural Heritage Program, 2007), there are sometimes also boom years in the number of reports of new populations. Because of reports of five new populations and a surge in numbers of plants at some previously-known sites, 2007 provided encouraging news for the conservation of the auriculate false foxglove [Agalinis auriculata (Michx.) Blake] in Missouri.