Changes in Leaf Functional Traits of the Endangered Plant Disanthus Cercidifolius Var
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Botanical Name Common Name
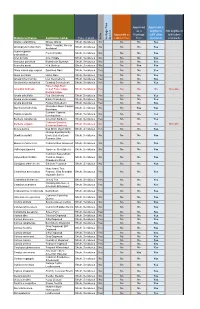
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Annual Benefit Plant Sale 2012
Annual Benefit Plant Sale 2012 Botanic Gardens COLLEGE OF AGRICULTURE & NATURAL RESOURCES Connect to nature Get inspired by wildflowers, naturalistic gardening and meadows in a whole new way with our seasonal garden tours. Enjoy an art class in the garden or learn about native plant gardening, conservation, and habitats by taking one of our classes. And if you can’t visit us, enroll in our new online distance learning program, Mt. Cuba Center Connect. Visit www.mtcubacenter.org to reserve a tour or sign up for a class. Two-Hour Guided Tours | $5 per person Spring Wildflower Tours April 12th – May 27th Summer Twilight Tours May 30th – July 26th 8th Annual Wildflower Celebration |Free th April 29 , 10am – 4pm Purple pitcherplant (Sarracenia purpurea) Greenville, DE P: 302.239.4244 www.mtcubacenter.org INSPIRATION x EDUCATION x CONSERVATION 2 2012 SPRING PLANT SALE CATALOG WEBSITE: http://ag.udel.edu/udbg/events/annualsale.html WELCOME We welcome you to the twentieth annual UDBG benefit plant sale. In addition to its role as the major source of funding for the UDBG, 2012 BENEFIT PLANT SALE CATALOG we hope it also serves as a major educational event for our members and the public. It presents an opportunity to learn about new plants and consider possibilities. We should always look for ways to expand and improve our knowledge about plants and this catalog offers possibilities to accomplish both. As always, we offer an in- depth look at a particular group of plants, this year the genus Camellia. The selection goes beyond offering various cultivars with differing flower color, to a more extensive exploration of the genus with particular emphasis on hardy selections and new hybrids Camellia ‘Autumn Spirit’. -

P. Aswathi.Cdr
T REPRO N DU LA C The International Journal of Plant Reproductive Biology 10(1) Jan., 2018, pp.65-68 P T I F V O E Y B T I DOI 10.14787/ijprb.2018 10.1.65-68 O E I L O C G O S I S E T S H Reproductive biology of Malabar tamarind (Garcinia gummi-gutta (L.) T Rob.: An endemic, medicinal and spice plant from Western Ghats P. Aswathi, K. Aswani and M. Sabu* Taxonomy Division, Department of Botany, University of Calicut, Kerala - 673 635, India *e-mail : [email protected] Received : 20.07.2017; Revised: 08.10.2017; Accepted and Published online: 01.01.2018 ABSTRACT Garcinia gummi-gutta, commonly called as Malabar tamarind or Kudampuli, is endemic to Western Ghats in India. The plant is a good source of medicinally important phytochemicals. Restricted distribution, habitat destruction and over harvesting for medicinal uses leads to declining of G. gummi-gutta population. Reproductive biology studies such as flowering phenology, flower morphology, pollen viability, stigma receptivity and pollination mechanism were carried out to suggest conservation measures. Garcinia gummi-gutta is dioecious, and the flowering season for both, male and female plants, occurred in March- April months. Anthesis of both male and female flowers and stigma receptivity were observed at evening hours. Small sized pollen grains were spherical in shape and bi, tetra or penta porate. About 4230 ± 602.7 pollen grains were produced per flower. On the day of anthesis high percentage of pollen grains were fertile. Stigma is wet type and broad umbrella-shaped. -

Botany-Illustrated-J.-Glimn-Lacy-P.-Kaufman-Springer-2006.Pdf
Janice Glimn-Lacy Peter B. Kaufman 6810 Shadow Brook Court Department of Molecular, Cellular, and Indianapolis, IN 46214-1901 Developmental Biology USA University of Michigan [email protected] Ann Arbor, MI 48109-1048 USA [email protected] Library of Congress Control Number: 2005935289 ISBN-10: 0-387-28870-8 eISBN: 0-387-28875-9 ISBN-13: 978-0387-28870-3 Printed on acid-free paper. C 2006 Janice Glimn-Lacy and Peter B. Kaufman All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, Inc., 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. Printed in the United States of America. (TB/MVY) 987654321 springer.com Preface This is a discovery book about plants. It is for everyone For those interested in the methods used and the interested in plants including high school and college/ sources of plant materials in the illustrations, an expla- university students, artists and scientific illustrators, nation follows. For a developmental series of drawings, senior citizens, wildlife biologists, ecologists, profes- there are several methods. -

Tree of the Year : Parrotia the Genus Parrotia Has Two Species: P
Tree of the Year : Parrotia The genus Parrotia has two species: P. persica, familiar to dendrologists, is very variable both in the wild and in cultivation; P. subaequalis is much rarer. SUSYN ANDREWS* has been researching these fascinating plants – with contributions from Colin Crosbie, Peter Del Tredici, Michael Dosmann, Gert Fortgens, Roy Lancaster and especially Mikinori Ogisu. One of the joys of putting together the “Trees of the Year” since 1993 is that you never know what gems of information you are going to unearth, whether on a letter attached to a herbarium specimen, a little known paper in an obscure journal, an observation on an herbarium label or a chance remark by someone in passing, will lead you down a convoluted trail of discovery. The highlight for this year has been the amassing of everything I could find on the enigmatic Parrotia subaequalis, the discovery of which has had dendrologists champing at the bit to possess a plant, myself included! 6 Introduction The Hamamelidaceae is one of the key areas for palaeobotanists and taxonomists to study the evolutionary history of the Hamamelids, especially the higher taxa. In his account of the family, H. Harms (1930) provided a comprehensive suprageneric classification for the Hamamelidaceae, recognising five subfamilies, Disanthoideae (1 genus), Hamamelidoideae (16 genera), Rhodoleioideae (1 genus), Bucklandioideae (1 genus) and Liquidambaroideae (2 genera). Although a shorter treatment was published by Schulze-Menze (1964), most workers still followed Harms. Since the mid-1960s, however, much new knowledge on the family has been amassed and this has led to new thinking on the intrafamilial relationships. -

Towards a Floristic Inventory of Bat Xat Nature Reserve, Vietnam
Wulfenia 27 (2020): 233 –250 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Towards a floristic inventory of Bat Xat Nature Reserve, Vietnam: Thirteen new national records of vascular plants Bui Hong Quang, Tran The Bach, Sangmi Eum, Do Van Hai, Nguyen Sinh Khang, Le Ngoc Han, Tran Duc Binh, Nguyen Thu Thuy, Vu Anh Thuong, Ngo Kien Trung, Ya-Ping Chen, Peter W. Fritsch, Chi-Ming Hu, Lu Thi Ngan, John A. N. Parnell, Alexander N. Sennikov, John R. I. Wood, Yi Yang, Andrey N. Kuznetsov, Svetlana P. Kuznetsova & Maxim S. Nuraliev Summary: The present study reports newly recorded species of vascular plants for the flora of Vietnam found in the recently established Bat Xat Nature Reserve in Lao Cai province, close to the border with Yunnan province of China. Thirteen species belonging to eleven families are reported: Acanthaceae (Strobilanthes helicta), Actinidiaceae (Actinidia melliana), Amaryllidaceae (Allium wallichii), Aquifoliaceae (Ilex fragilis), Asteraceae (Melanoseris leiolepis), Begoniaceae (Begonia yuii ), Lamiaceae (Callicarpa giraldii, Clerodendrum peii, Scutellaria macrosiphon), Lentibulariaceae (Utricularia spinomarginata), Primulaceae (Lysimachia septemfida), Pteridaceae (Aleuritopteris chrysophylla) and Symplocaceae (Symplocos glandulifera). Some of these species are additionally reported from the neighbouring Hoang Lien National Park. For each species, information on its habitat, phenology, distribution and studied specimens is provided along with the photographs of the reported findings. Keywords: Hoang Lien National Park, Indochinese Peninsula, Lao Cai province, Southeast Asia, Vietnam-China border area, Y Ty area Bat Xat Nature Reserve is located in Bat Xat district of Lao Cai province, northwestern Vietnam. It was established in 2016 by the Decision No.1954/QD-UBND of the President of Lao Cai province “On the establishment of Bat Xat Nature Reserve” (DARD 2016) in order to conserve primeval forest ecosystems in the highlands, and in particular, the rare and endangered species of flora and fauna typical of Hoang Lien Son mountain region. -

Fall 2007 – Vol
FRIENDS OF THE JC RAULSTON ARBORETUM NEWSLETTER FALL 2007 – VOL. 11, NO. 2 WORDS FROM THE DIRECTOR Director’s Letter Arborfest annually at the JCRA. All of us on the staff of the JCRA will miss her friendship, her smile, and her passion. Frankie, thank you for all you have By Denny Werner, Ph.D., Director contributed. We wish you the best. Welcome from the JC Raulston Arboretum. I am pleased to announce that Barbara Kennedy I am writing this message content in the assumed the position of JCRA volunteer coordinator knowledge that, for the first time since I effective July 30, 2007. Barbara has been a volunteer assumed the position of director, the JCRA at the JCRA since 2001, and has assumed many roles is fully staffed. Mark Weathington, our in her volunteer services over the years, including tour new assistant director, joined us on July guide and leader of the Tuesday morning 23, and he has “hit the ground roving gardening group. She earned a running.” Mark’s knowledge of plants is remarkable, degree in horticulture from NC State and his passion for the JCRA and its collections is University in 2003. She also has a B.S. infectious. All of us on the staff look forward to his in business education from the College of future contributions to the gardens and programs. One New Jersey, and a M.S. in business from of Mark’s first initiatives upon arriving was to resume the the Georgia Institute of Technology. monthly Plantsmen’s Tours, historically a popular program at the JCRA. -

DS Govt Cov Idaho2003
2003 AND SUCCEEDING CROP YEARS FEDERAL CROP INSURANCE CORPORATION ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE NURSERY CROP INSURANCE PROGRAM • IDAHO • OREGON • WASHINGTON The price for each plant and size listed in the Eligible Plant List and Plant Price Schedule is your lowest wholesale price, as determined from your wholesale catalogs or price lists submitted in accordance with the Special Provisions, not to exceed the maximum price limits included in this Schedule. Insurable plants damaged prior to the attachment of insurance coverage will be insured at a reduced value until such plants have fully recovered from damage. CONTENTS INTRODUCTION Crop Insurance Nomenclature Format Determining Eligible Plant List Price of Unlisted Cultivars Crop Type and Optional Units Storage Keys Hardiness Zone Designations Container Insurable Hardiness Zones Field Grown Minimum Hardiness Zones Plant Size SOFTWARE AVAILABILITY System Requirements Sample Report INSURANCE PRICE CALCULATION Examples of Price Calculation Crop Type Base Price Tables ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE APPENDIX A County Hardiness Zones B Storage Keys C Insurance Price Calculation Worksheet D Container Volume Calculation Worksheet E FCIC Container Definitions The DataScape Guide to Commercial Nomenclature is used in this document by the Federal Crop Insurance Corporation (FCIC), an agency of the United States Department of Agriculture (USDA), with permission. Permission is given to use or reproduce this Eligible Plant List and Plant Price Schedule for purposes of administering the Federal Crop Insurance Corporation’s Nursery Insurance program only. The DataScape Guide to Commercial Nomenclature is published in electronic format by DataScape, LLC, 1250 South Grove Avenue, Suite 302, Barrington, IL 60010. -

Polly Hill Arboretum Plant Collection Inventory March 14, 2011 *See
Polly Hill Arboretum Plant Collection Inventory March 14, 2011 Accession # Name COMMON_NAME Received As Location* Source 2006-21*C Abies concolor White Fir Plant LMB WEST Fragosa Landscape 93-017*A Abies concolor White Fir Seedling ARB-CTR Wavecrest Nursery 93-017*C Abies concolor White Fir Seedling WFW,N1/2 Wavecrest Nursery 2003-135*A Abies fargesii Farges Fir Plant N Morris Arboretum 92-023-02*B Abies firma Japanese Fir Seed CR5 American Conifer Soc. 82-097*A Abies holophylla Manchurian Fir Seedling NORTHFLDW Morris Arboretum 73-095*A Abies koreana Korean Fir Plant CR4 US Dept. of Agriculture 73-095*B Abies koreana Korean Fir Plant ARB-W US Dept. of Agriculture 97-020*A Abies koreana Korean Fir Rooted Cutting CR2 Jane Platt 2004-289*A Abies koreana 'Silberlocke' Korean Fir Plant CR1 Maggie Sibert 59-040-01*A Abies lasiocarpa 'Martha's Vineyard' Arizona Fir Seed ARB-E Longwood Gardens 59-040-01*B Abies lasiocarpa 'Martha's Vineyard' Arizona Fir Seed WFN,S.SIDE Longwood Gardens 64-024*E Abies lasiocarpa var. arizonica Subalpine Fir Seedling NORTHFLDE C. E. Heit 2006-275*A Abies mariesii Maries Fir Seedling LNNE6 Morris Arboretum 2004-226*A Abies nephrolepis Khingan Fir Plant CR4 Morris Arboretum 2009-34*B Abies nordmanniana Nordmann Fir Plant LNNE8 Morris Arboretum 62-019*A Abies nordmanniana Nordmann Fir Graft CR3 Hess Nursery 62-019*B Abies nordmanniana Nordmann Fir Graft ARB-CTR Hess Nursery 62-019*C Abies nordmanniana Nordmann Fir Graft CR3 Hess Nursery 62-028*A Abies nordmanniana Nordmann Fir Plant ARB-W Critchfield Tree Fm 95-029*A Abies nordmanniana Nordmann Fir Seedling NORTHFLDN Polly Hill Arboretum 86-046*A Abies nordmanniana ssp. -

Monophyly and Relationships of the Enigmatic Family Peridiscaceae
TAXON 56 (1) • February 2007: 65–73 Soltis & al. • Monophyly and relationships of Peridiscaceae Monophyly and relationships of the enigmatic family Peridiscaceae Douglas E. Soltis1, Joshua W. Clayton1, Charles C. Davis2, Matthew A. Gitzendanner1, Martin Cheek3, Vincent Savolainen3, André M. Amorim4 & Pamela S. Soltis5 1 Department of Botany, University of Florida, Gainesville, Florida 32611, U.S.A. [email protected] (author for correspondence) 2 Harvard University Herbaria, Department of Organismic and Evolutionary Biology, Cambridge, Massachusetts 02138, U.S.A. 3 Royal Botanic Gardens, Kew, Richmond TW9 3DS, U.K. 4 Departamento de Ciências Biológicas, Universidade Estadual de Santa Cruz, Illhéus, 46.650-000, Bahia, Brazil 5 Florida Museum of Natural History, University of Florida, Gainesville, Florida 32611, U.S.A. Peridiscaceae, comprising Peridiscus, Soyauxia, and Whittonia, are an enigmatic angiosperm family of uncertain composition and placement. Although some have placed Soyauxia in other families (e.g., Flacourtiaceae, Medusandraceae), rather than in Peridiscaceae, sequence data for five genes (material of Whittonia could not be obtained) provide strong support for a clade of Soyauxia and Peridiscus. This evidence, combined with the strong morphological similarity of Peridiscus and Whittonia, support a monophyletic Peridiscaceae of three genera. Molecular analyses of a three-gene (rbcL, atpB, 18S rDNA) dataset for 569 taxa indicate that Peridiscus + Soyauxia together with Daphniphyllaceae form a clade that is sister to the rest of Saxifragales. Maximum likelihood and Bayesian analyses of Saxifragales using a five-gene (rbcL, atpB, matK, 18S rDNA, 26S rDNA) dataset place Peridiscaceae (posterior probability of 1.00) Peridiscaceae as sister to the remainder of Saxifragales, albeit without high posterior probability (pp = 0.78). -

Disanthus Cercidifolius Subsp. Longipes (Hamamelidaceae) Based on AFLP Analysis
Genetic Variability and Population Structure of Disanthus cercidifolius subsp. longipes (Hamamelidaceae) Based on AFLP Analysis Yi Yu1, Qiang Fan1, Rujiang Shen1, Wei Guo3, Jianhua Jin1, Dafang Cui2*, Wenbo Liao1* 1 Guangdong Key Laboratory of Plant Resources and Key Laboratory of Biodiversity Dynamics and Conservation of Guangdong Higher Education Institutes, School of Life Sciences, Sun Yat-Sen University, Guangzhou, China, 2 College of Forestry, South China Agriculture University, Guangzhou, China, 3 Department of Horticulture and Landscape Architecture, Zhongkai University of Agriculture and Engineering, Guangzhou, China Abstract Disanthus cercidifolius subsp. longipes is an endangered species in China. Genetic diversity and structure analysis of this species was investigated using amplified fragments length polymorphism (AFLP) fingerprinting. Nei’s gene diversity ranged from 0.1290 to 0.1394. The AMOVA indicated that 75.06% of variation was distributed within populations, while the between-group component 5.04% was smaller than the between populations-within-group component 19.90%. Significant genetic differentiation was detected between populations. Genetic and geographical distances were not correlated. PCA and genetic structure analysis showed that populations from East China were together with those of the Nanling Range. These patterns of genetic diversity and levels of genetic variation may be the result of D. c. subsp. longipes restricted to several isolated habitats and ‘‘excess flowers production, but little fruit set’’. It is necessary to protect all existing populations of D. c. subsp. longipes in order to preserve as much genetic variation as possible. Citation: Yu Y, Fan Q, Shen R, Guo W, Jin J, et al. (2014) Genetic Variability and Population Structure of Disanthus cercidifolius subsp. -

Functional Integration of Floral Plant Traits: Shape and Symmetry, Optical Signal, Reward and Reproduction in the Angiosperm Flower
Functional Integration of Floral Plant Traits: Shape and Symmetry, Optical Signal, Reward and Reproduction in the Angiosperm Flower Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Andreas Wilhelm Mues aus Kirchhellen Bonn, den 20. Januar 2020 1 2 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn Erstgutachter: Prof. Dr. Maximilian Weigend, Universität Bonn Zweitgutachter: Prof. Dr. Eberhard Fischer, Universität Koblenz Tag der Promotion: 30. April 2020 Erscheinungsjahr: 2020 3 4 Acknowledgements I thank Prof. Dr. Maximilian Weigend, supervisor, for his guidance and support, and for giving me the opportunity to study the holistic subject of floral functional integration and plant-animal interaction. I am grateful for the experience and for the research agendas he entrusted to me: Working with the extensive Living Collections of Bonn Botanical Gardens was an honour, and I have learned a lot. I thank Prof. Dr. Eberhard Fisher, for agreeing to be my second supervisor, his advice and our shared passion for the plant world. I would like to thank many people of the Nees Institute and Bonn Botanical Gardens who contributed to this work and who gave me good memories of my years of study: I thank Lisabeth Hoff, Tianjun Liu, Luisa Sophie Nicolin and Simon Brauwers for their contribution in collecting shares of the raw data together with me, and for being eager students – especially counting pollen and ovule numbers and measuring nectar reward was a test of patience sometimes, and we have counted and measured a lot … Thank you! Special thanks go to Gardeners of the Bonn Botanical Gardens, for their constant support throughout the years, their love for the plant world in general and their commitment and care for the Living Collection: Klaus Mahlberg (Streptocarpus), Birgit Emde (carnivorous plants), Klaus Bahr (Geraniales), Bernd Reinken and Klaus Michael Neumann.