Oocyte Cryopreservation Packet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -
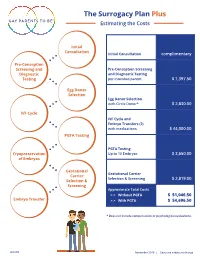
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

Social Freezing: Pressing Pause on Fertility
International Journal of Environmental Research and Public Health Review Social Freezing: Pressing Pause on Fertility Valentin Nicolae Varlas 1,2 , Roxana Georgiana Bors 1,2, Dragos Albu 1,2, Ovidiu Nicolae Penes 3,*, Bogdana Adriana Nasui 4,* , Claudia Mehedintu 5 and Anca Lucia Pop 6 1 Department of Obstetrics and Gynaecology, Filantropia Clinical Hospital, 011171 Bucharest, Romania; [email protected] (V.N.V.); [email protected] (R.G.B.); [email protected] (D.A.) 2 Department of Obstetrics and Gynaecology, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 3 Department of Intensive Care, University Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 4 Department of Community Health, “Iuliu Hat, ieganu” University of Medicine and Pharmacy, 6 Louis Pasteur Street, 400349 Cluj-Napoca, Romania 5 Department of Obstetrics and Gynaecology, Nicolae Malaxa Clinical Hospital, 020346 Bucharest, Romania; [email protected] 6 Department of Clinical Laboratory, Food Safety, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia Street, 020945 Bucharest, Romania; [email protected] * Correspondence: [email protected] (O.N.P.); [email protected] (B.A.N.) Abstract: Increasing numbers of women are undergoing oocyte or tissue cryopreservation for medical or social reasons to increase their chances of having genetic children. Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. The purpose of this study was to summarize recent findings focusing on the challenges of elective egg freezing. -

Let She Who Has the Womb Speak: Regulating the Use of Human Oocyte Cryopreservation to the Detriment of Older Women
Arkansas Law Review Volume 72 Number 3 Article 2 February 2020 Let She Who has the Womb Speak: Regulating the use of Human Oocyte Cryopreservation to the Detriment of Older Women Browne C. Lewis Cleveland State University Follow this and additional works at: https://scholarworks.uark.edu/alr Part of the Family Law Commons Recommended Citation Browne C. Lewis, Let She Who has the Womb Speak: Regulating the use of Human Oocyte Cryopreservation to the Detriment of Older Women, 72 Ark. L. Rev. 597 (2020). Available at: https://scholarworks.uark.edu/alr/vol72/iss3/2 This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Arkansas Law Review by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. LET SHE WHO HAS THE WOMB SPEAK: REGULATING THE USE OF HUMAN OOCYTE CRYOPRESERVATION TO THE DETRIMENT OF OLDER WOMEN Browne C. Lewis INTRODUCTION “Inequality starts in the womb.”1 When it comes to childbearing, advances in assisted reproductive technology (ART) may negate the veracity of this quote. In her autobiography Becoming,2 former First Lady Michelle Obama discusses her struggles with infertility.3 Mrs. Obama’s difficulty getting pregnant may have stemmed from the fact that she postponed motherhood to focus on her career as a high-powered attorney.4 At that time, for Mrs. Obama and women of her generation, the focus was on pregnancy prevention instead of procreation preservation.5 Women feared being placed on the “mommy track,”6 so they waited to have children until after they had achieved success in their careers.7 Some of those women paid B.A., Grambling State University, M.P.P., Humphrey Institute; J.D., University of Minnesota School of Law; University of Houston Law Center. -

Specific Protocols of Controlled Ovarian Stimulation for Oocyte Cryopreservation in Breast Cancer Patients
OVARIAN STIMULATION IN BREAST CANCERORIGINAL PATIENTS, CavagnaARTICLE et al. Specific protocols of controlled ovarian stimulation for oocyte cryopreservation in breast cancer patients † F. Cavagna MD,* A. Pontes MD, M. Cavagna MD,* A. Dzik MD,* N.F. Donadio MD,* R. Portela BSc,* M.T. Nagai MD,* and L.H. Gebrim MD* ABSTRACT Background Fertility preservation is an important concern in breast cancer patients. In the present investigation, we set out to create a specific protocol of controlled ovarian stimulation (cos) for oocyte cryopreservation in breast cancer patients. Methods From November 2014 to December 2016, 109 patients were studied. The patients were assigned to a specific random-start ovarian stimulation protocol for oocyte cryopreservation. The endpoints were the numbers of oocytes retrieved and of mature oocytes cryopreserved, the total number of days of ovarian stimulation, the total dose of gonadotropin administered, and the estradiol level on the day of the trigger. Results Mean age in this cohort was 31.27 ± 4.23 years. The average duration of cos was 10.0 ± 1.39 days. The mean number of oocytes collected was 11.62 ± 7.96 and the mean number of vitrified oocytes was 9.60 ± 6.87. The mean estradiol concentration on triggering day was 706.30 ± 450.48 pg/mL, and the mean dose of gonadotropins administered was 2610.00 ± 716.51 IU. When comparing outcomes by phase of the cycle in which cos was commenced, we observed no significant differences in the numbers of oocytes collected and vitrified, the length of ovarian stimulation, and the estradiol level on trigger day. -

Oocyte Warming and Insemination Consent Form
Oocyte Warming and Insemination Process, Risk, and Consent While embryos and sperm have been frozen and thawed with good results for many years, eggs have proved much more difficult to manage. Newer egg freezing methods have been more successful, at least in younger women, the main population in which the techniques have been studied. Egg freezing takes place by one of two methods: a slow freeze protocol, or a different “flash freeze” method known as vitrification. USF IVF uses the newer vitrification technology exclusively. There are two sources of frozen eggs. Some women may have chosen to have had their eggs frozen for the purpose of fertility preservation at some time prior to warming. Other women may have obtained frozen eggs donated by an egg donor. This consent reviews the Oocyte warming process from start to finish, including the risks that this treatment might pose to you and your offspring. While best efforts have been made to disclose all known risks, there may be risks of oocyte freezing, subsequent warming, and the IVF process, that are not yet clarified or even suspected at the time of this writing. Oocyte warming and subsequent embryo transfer typically includes the following steps or procedures: Medications to prepare the uterus to receive embryos Oocyte warming Insemination of eggs that have survived warming with sperm, using intracytoplasmic sperm injection (ICSI) Culture of any resulting fertilized eggs (embryos) Placement (transfer) of one or more embryos into the uterus Support of the uterine lining with hormones to permit and sustain pregnancy In certain cases, these additional procedures can be employed: Assisted hatching of embryos to potentially increase the chance of embryo attachment (implantation) Cryopreservation (freezing) of embryos Is pregnancy achieved as successfully with frozen eggs as with fresh eggs? As mentioned, most studies have looked at success rates using either donor eggs or women who produced larger numbers of eggs. -

MG Infertility Services Commercial
Infertility Services — Commercial Last Review Date: June 11, 2021 Number: MG.MM.ME.53f Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth the clinical evidence that the patient meets the criteria for the treatment or surgical procedure. Without this documentation and information, EmblemHealth will not be able to properly review the request for prior authorization. The clinical review criteria expressed below reflects how EmblemHealth determines whether certain services or supplies are medically necessary. EmblemHealth established the clinical review criteria based upon a review of currently available clinical information (including clinical outcome studies in the peer reviewed published medical literature, regulatory status of the technology, evidence-based guidelines of public health and health research agencies, evidence-based guidelines and positions of leading national health professional organizations, views of physicians practicing in relevant clinical areas, and other relevant factors). EmblemHealth expressly reserves the right to revise these conclusions as clinical information changes and welcomes further relevant information. Each benefit program defines which services are covered. The conclusion that a particular service or supply is medically necessary does not constitute a representation or warranty that this service or supply is covered and/or paid for by EmblemHealth, as some programs exclude coverage for services or supplies that EmblemHealth considers medically necessary. If there is a discrepancy between this guideline and a member's benefits program, the benefits program will govern. In addition, coverage may be mandated by applicable legal requirements of a state, the Federal Government or the Centers for Medicare & Medicaid Services (CMS) for Medicare and Medicaid members. -

Planned Oocyte Cryopreservation for Women Seeking to Preserve Future Reproductive Potential: an Ethics Committee Opinion
Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an Ethics Committee opinion Ethics Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Planned oocyte cryopreservation (‘‘planned OC’’) is an emerging but ethically permissible procedure that may help women avoid future infertility. Because planned OC is new and evolving, it is essential that women who are considering using it be informed about the un- certainties regarding its efficacy and long-term effects. (Fertil SterilÒ 2018;110:1022–8. Ó2018 by American Society for Reproductive Medicine.) Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility- and-sterility/posts/37565-26808 KEY POINTS informed decision-making. Pro- dotoxic medical treatment, the chance viders should disclose their own to have biologically related children in For women who want to try to pro- clinic-specific statistics, or lack the future. The history of cryopreserva- tect against future infertility due to thereof, for successful freeze-thaw tion of sperm, embryos, and oocytes is reproductive aging or other causes, and for live birth. Patients should set forth in the ASRM Practice Commit- advance oocyte cryopreservation be informed that medical benefits tee document, ‘‘Mature oocyte preser- ‘‘ ’’ ( OC ) is ethically permissible. The are uncertain and harms that are vation: a guideline’’ (1). While the first Ethics Committee will refer to this not fully understood may emerge human birth from a previously frozen ‘‘ procedure as planned oocyte cryo- from planned OC. oocyte occurred in 1986, the more ’’ ‘‘ ’’ preservation or planned OC. -

Optimizing Human Oocyte Cryopreservation for Fertility Preservation Patients: Should We Mature Then Freeze Or Freeze Then Mature?
ORIGINAL ARTICLES: FERTILITY PRESERVATION Optimizing human oocyte cryopreservation for fertility preservation patients: should we mature then freeze or freeze then mature? Joseph A. Lee, B.S.,a Jason Barritt, Ph.D.,a,b Rose Marie Moschini, B.S.,a Richard E. Slifkin, B.S.,a and Alan B. Copperman, M.D.a,b a Reproductive Medicine Associates of New York, and b Department of Obstetrics, Gynecology and Reproductive Science, Mount Sinai School of Medicine, New York, New York Objective: To evaluate the maturation and post-thaw survival rates of immature oocytes to determine whether in vitro maturation (IVM) should be attempted prior to or after cryopreservation. Design: Nonrandomized observational study. Setting: Private academic and clinical reproductive center. Patient(s): Patients (n ¼ 71) who donated immature unusable oocytes after vaginal oocyte retrieval (VOR) after undergoing controlled ovarian hyperstimulation using a standard GnRH antagonist protocol. Intervention(s): Germinal vesicle (GV), metaphase I (MI), and metaphase II (MII) oocytes (n ¼ 175) were obtained from consenting IVF patients for fresh IVM, post-thaw IVM, or control group. In the fresh IVM group, GV- and MI- stage oocytes (n ¼ 69) were cultured for 24 hours, matured in vitro (IVM-MII), cryopreserved, thawed, and evaluated for survival. In the post-thaw IVM group, GV- and MI- stage oocytes (n ¼ 27) were frozen on day 0, thawed, evaluated for survival, and cultured for 24-hour IVM. MII donor oocytes (n ¼ 79) were cryopreserved and thawed as a control. Main Outcome Measure(s): Survival postfreeze and oocyte development to the MII stage was analyzed using a c2 analysis. -

Fertility Preservation (Oocyte Freezing Etc...) PGD, PGS
Other laboratory technologies: Fertility preservation (oocyte freezing etc...) PGD, PGS... M.C. Magli, L. Gianaroli S.I.S.ME.R. Reproductive Medicine Unit - Via Mazzini, 12 - 40138 Bologna Italy [email protected] S.I.S.ME.R. ISO 9001:2008 LEARNING OBJECTIVES 1) Embryo / 2pn cryopreservation 2) Oocyte cryopreservation 3) PGD 4) PGS S.I.S.ME.R. ISO 9001:2008 Cryopreservation EMBRYOS / 2 pn - Reasons for cryopreservation of human embryos • To increase efficiency of ART • To reduce multiple pregnancies • To transfer in natural cycle • Fer tility preserva tion S.I.S.ME.R. ISO 9001:2008 Cryopreservation EMBRYOS / 2 pn - Cryopreservation policy 1) cryopreserving before morphology becomes a substantial factor: two pronucleate stage 2) optimizing fresh transfer by selecting the morphologically best embryos to be transferred; cryopreservation of spare cleavage stage embryos or blastocysts Which embryos to cryopreserve? Which technique to use? S.I.S.ME.R. ISO 9001:2008 Cryopreservation Embryos There is a significant difference in the clinical outcome from fresh and frozen cycles. *P<0.001 Is the technique itself detrimental? www.eshre.eu>specialty groups>special interest group Embryology>archive>Athens2009>Edgar S.I.S.ME.R. ISO 9001:2008 Cryopreservation Embryos Factors impacting could be: - Quality of embryos prior to cryopreservation - Biological consequences of freezing/thawing - Efficiency of methodology S.I.S.ME.R. ISO 9001:2008 Cryopreservation Quality of embryos prior to cryopreservation Fresh vs. equivalent cryo embryos NO DIFFERENCE S.I.S.ME.R. ISO 9001:2008 Cryopreservation Biological consequences of freezing/thawing Possible biological consequences of embryo cryopreservation !! - Cell loss !! - Arrested/compromised development Cell loss tends to reduce the implantation potential S.I.S.ME.R.