Social Freezing: Pressing Pause on Fertility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Romania Redivivus
alexander clapp ROMANIA REDIVIVUS nce the badlands of neoliberal Europe, Romania has become its bustling frontier. A post-communist mafia state that was cast to the bottom of the European heap by opinion- makers sixteen years ago is now billed as the success story Oof eu expansion.1 Its growth rate at nearly 6 per cent is the highest on the continent, albeit boosted by fiscal largesse.2 In Bucharest more politicians have been put in jail for corruption over the past decade than have been convicted in the rest of Eastern Europe put together. Romania causes Brussels and Berlin almost none of the headaches inflicted by the Visegrád Group—Czechia, Hungary, Poland, Slovakia— which in 1993 declined to accept Romania as a peer and collectively entered the European Union three years before it. Romanians con- sistently rank among the most Europhile people in the Union.3 An anti-eu party has never appeared on a Romanian ballot, much less in the parliament. Scattered political appeals to unsavoury interwar traditions—Legionnairism, Greater Romanianism—attract fewer voters than do far-right movements across most of Western Europe. The two million Magyars of Transylvania, one of Europe’s largest minorities, have become a model for inter-ethnic relations after a time when the park benches of Cluj were gilded in the Romanian tricolore to remind every- one where they were. Indeed, perhaps the aptest symbol of Romania’s place in Europe today is the man who sits in the Presidential Palace of Cotroceni in Bucharest. Klaus Iohannis—a former physics teacher at a high school in Sibiu, once Hermannstadt—is an ethnic German head- ing a state that, a generation ago, was shipping hundreds of thousands of its ‘Saxons’ ‘back’ to Bonn at 4,000–10,000 Deutschmarks a head. -

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

Creating an Artificial 3-Dimensional Ovarian Follicle Culture System
micromachines Article Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System Mae W. Healy 1,2, Shelley N. Dolitsky 1, Maria Villancio-Wolter 3, Meera Raghavan 3, Alexandra R. Tillman 3 , Nicole Y. Morgan 3, Alan H. DeCherney 1, Solji Park 1,*,† and Erin F. Wolff 1,4,† 1 Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.W.H.); [email protected] (S.N.D.); [email protected] (A.H.D.); [email protected] (E.F.W.) 2 Department of Obstetrics and Gynecology, Walter Reed National Military Medical Center, Bethesda, MD 20889, USA 3 Trans-NIH Shared Resource on Biomedical Engineering and Physical Science, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.V.-W.); [email protected] (M.R.); [email protected] (A.R.T.); [email protected] (N.Y.M.) 4 Pelex, Inc., McLean, VA 22101, USA * Correspondence: [email protected] † Solji Park and Erin F. Wolff are co-senior authors. Abstract: We hypothesized that the creation of a 3-dimensional ovarian follicle, with embedded gran- ulosa and theca cells, would better mimic the environment necessary to support early oocytes, both structurally and hormonally. Using a microfluidic system with controlled flow rates, 3-dimensional Citation: Healy, M.W.; Dolitsky, S.N.; two-layer (core and shell) capsules were created. The core consists of murine granulosa cells in Villancio-Wolter, M.; Raghavan, M.; 0.8 mg/mL collagen + 0.05% alginate, while the shell is composed of murine theca cells suspended Tillman, A.R.; Morgan, N.Y.; in 2% alginate. -

Educational Objectives Current IVF Practice
2/23/2015 Update from the IVF Lab: Preimplantation Genetic Testing Janet McLaren Bouknight, MD Reproductive Endocrinology and Infertility Department of Obstetrics & Gynecology Educational Objectives • Gain familiarity with the procedures and pregnancy outcomes of current in vitro fertilization practices. • Understand the application of Preimplantation Genetic Diagnosis (PGD) for single‐gene disorders. • Appreciate the role of Preimplantation Genetic Screening (PGS) in maximizing IVF cycle efficiency and encouraging elective single embryo transfer. Current IVF Practice 1 2/23/2015 Current IVF Practice 150,000 cycles 65,000 livebirths Current IVF Practice Current IVF Practice All SART Clinics 2012 <35 35‐37 38‐40 41‐42 >42 Pregnancy Rate 47% 38% 30% 20% 9% Live Birth Rate 41% 31% 22% 12% 4% # embryos transfer 1.9 2.0 2.4 2.9 2.9 % Twins 30% 25% 20% 13% 9% • What are the advanced that have contributed to an increase in success in the IVF Lab? • Improved lab conditions • Extended embryo culture • Improvements in embryo cryopreservation 2 2/23/2015 Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions 3 2/23/2015 Current IVF Practice: Extended Embryo Culture Day 3: Cleavage Stage Day 5: Blastocyst Stage • 8‐cells • 200‐300 cells • 30% implantation rate • 50% implantation rate Increased live birth rates seen in “good prognosis” patients who undergo a Day 5 transfer Current IVF Practice: Extended Embryo Culture Current IVF Practice: Extended Embryo Culture • Supports blastocyst transfer for “good prognosis” patients • Opportunity to consider single embryo transfer • May be associated with a small increased risk of monozygotic twinning 4 2/23/2015 Current IVF Practice: Embryo Cryopreservation Cycles • Improvements in freezing techniques (vitrification) have increased success of cryopreserved embryos. -
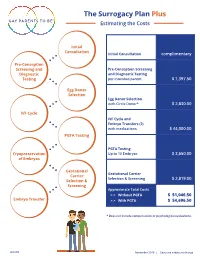
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

Romania: List of Archival Holdings at the Hoover Institution Archives
Romania: List of Archival Holdings at the Hoover Institution Archives Lista colectiilor din arhive Maica Alexandra (Principesa Ileana) Fiica regelui Ferdinand si a Reginei Maria, Stareta a Manastirii ‘Schimbarea la fata’, Elwood City, Pennsylvania Colectia sa include manuscrise, fotografii si corespondenta cu privire la Familia Regala Romana si Biserica Ortodoxa Romana din SUA, cit si fotocopii ale corespondentei particulare a Reginei Maria. (Scrisorile sale originale se gasesc si in colectii ca Breed, sau Duca). Antoniade, Constantin Diplomat roman. Colectia sa include o scrisoare de autorizare din partea lui Iuliu Maniu si Dinu Bratianu pentru formarea contactelor in strainatate ale PNT si PNL. Bacon, Walter Political scientist american. Colectia sa include documente referitoare la Titulescu. Bayne, Joseph B. Chirurg american ce a lucrat in cadrul Crucii Rosii romane. Colectia sa include fotografii si fotocopii ale unor documente referitoare la spitalele civile si militare din Romania in timpul Primului Razboi Mondial. Beane, James Locotenent colonel, Fortele Aeriene SUA. Colectia sa include documente referitoare la conditiile de viata aleprizonierilor americani in Romania, in timpul celui de-al Doilea Razboi Mondial. Berthelot, Henry General in armata franceza. Colectia sa include fotocopii ale jurnalului personal cuprinzind informatii referitoare la misiunile militare franceze in Romania in timpul Primului Razboi Mondial. Blank si Bunescu Cele doua colectii includ colectii de ziare, buletine, note si pamflete referitoare la Romania in secolul XX. Bossy, Raul Diplomat roman, ambasador in Austria (1931-1934), Finlanda (1934-1936), Ungaria (1936-1939), Italia (1939-1940) si Germania (1940-1941). Colectia sa include memorii referitoare la Romania antebelica si situatia emigratiei 1 romanesti postbelice. -

Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity
International Journal of Molecular Sciences Review Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity Hyun-Woong Cho, Sanghoon Lee * , Kyung-Jin Min , Jin Hwa Hong , Jae Yun Song, Jae Kwan Lee , Nak Woo Lee and Tak Kim Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul 02841, Korea; [email protected] (H.-W.C.); [email protected] (K.-J.M.); [email protected] (J.H.H.); [email protected] (J.Y.S.); [email protected] (J.K.L.); [email protected] (N.W.L.); [email protected] (T.K.) * Correspondence: [email protected]; Tel.: +82-2-920-6773 Received: 9 October 2020; Accepted: 20 October 2020; Published: 21 October 2020 Abstract: Due to improvements in chemotherapeutic agents, cancer treatment efficacy and cancer patient survival rates have greatly improved, but unfortunately gonadal damage remains a major complication. Gonadotoxic chemotherapy, including alkylating agents during reproductive age, can lead to iatrogenic premature ovarian insufficiency (POI), and loss of fertility. In recent years, the demand for fertility preservation has increased dramatically among female cancer patients. Currently, embryo and oocyte cryopreservation are the only established options for fertility preservation in women. However, there is growing evidence for other experimental techniques including ovarian tissue cryopreservation, oocyte in vitro maturation, artificial ovaries, stem cell technologies, and ovarian suppression. To prevent fertility loss in women with cancer, individualized fertility -

Approaches and Technologies in Male Fertility Preservation
International Journal of Molecular Sciences Review Approaches and Technologies in Male Fertility Preservation Mahmoud Huleihel 1,2,* and Eitan Lunenfeld 2,3,4 1 The Shraga Segal Department of Microbiology, Immunology and Genetics, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel 2 The Center of Advanced Research and Education in Reproduction (CARER), Ben-Gurion University of the Negev, Beer Sheva 84105, Israel; lunenfl[email protected] 3 Department of OB/GYN, Soroka Medical Center, Beer Sheva 8410501, Israel 4 Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel * Correspondence: [email protected]; Tel.: +972-86479959 Received: 28 May 2020; Accepted: 29 July 2020; Published: 31 July 2020 Abstract: Male fertility preservation is required when treatment with an aggressive chemo-/-radiotherapy, which may lead to irreversible sterility. Due to new and efficient protocols of cancer treatments, surviving rates are more than 80%. Thus, these patients are looking forward to family life and fathering their own biological children after treatments. Whereas adult men can cryopreserve their sperm for future use in assistance reproductive technologies (ART), this is not an option in prepubertal boys who cannot produce sperm at this age. In this review, we summarize the different technologies for male fertility preservation with emphasize on prepubertal, which have already been examined and/or demonstrated in vivo and/or in vitro using animal models and, in some cases, using human tissues. We discuss the limitation of these technologies for use in human fertility preservation. This update review can assist physicians and patients who are scheduled for aggressive chemo-/radiotherapy, specifically prepubertal males and their parents who need to know about the risks of the treatment on their future fertility and the possible present option of fertility preservation. -

Preservation of Fertility in Patients with Cancer (Review)
ONCOLOGY REPORTS 41: 2607-2614, 2019 Preservation of fertility in patients with cancer (Review) SOFÍA DEL-POZO-LÉRIDA1, CRISTINA SALVADOR2, FINA MARTÍNEZ-SOLER3, AVELINA TORTOSA3, MANUEL PERUCHO4,5 and PEPITA GIMÉNEZ-BONAFÉ1 1Department of Physiological Sciences, Physiology Unit, Faculty of Medicine and Health Sciences, Bellvitge Campus, Universitat de Barcelona, IDIBELL, L'Hospitalet del Llobregat, 08907 Barcelona; 2Department of Gynecology, Gynecological Endocrinology and Reproduction Unit, Hospital Universitari Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona; 3Department of Basic Nursing, Faculty of Medicine and Health Sciences, Universitat de Barcelona, IDIBELL, L' Hospital et del Llobregat, 08907 Barcelona, Spain; 4Sanford Burnham Prebys Medical Discovery Institute (SBP), La Jolla, CA 92037, USA; 5Program of Predictive and Personalized Medicine of Cancer (PMPPC), of the Research Institute Germans Trias i Pujol (IGTP), Badalona, 08916 Barcelona, Spain Received January 18, 2019; Accepted March 6, 2019 DOI: 10.3892/or.2019.7063 Abstract. Survival rates in oncological patients have been techniques evaluated. Emerging techniques are promising, steadily increasing in recent years due to the greater effec- such as the cryopreservation in orthotopic models of ovarian tiveness of novel oncological treatments, such as radio- and or testicle tissues, artificial ovaries, or in vitro culture prior chemotherapy. However, these treatments impair the reproduc- to the autotransplantation of cryopreserved tissues. However, tive ability of patients, and may cause premature ovarian failure oocyte vitrification for female patients and sperm banking for in females and azoospermia in males. Fertility preservation male patients are considered the first line fertility preserva- in both female and male oncological patients is nowadays tion option at the present time for cancer patients undergoing possible and should be integrated as part of the oncological treatment. -

Conference Abstracts
Conference abstracts. IV International Symposium on Animal Biology of Reproduction, Oct. 17-20, 2012, Campinas, SP, Brazil. Advances in goat artificial ovary J.R. Figueiredo Laboratory of Manipulation of Oocytes and Preantral Follicles (LAMOFOPA), Faculty of Veterinary, State University of Ceara, Fortaleza, Ceara, Brazil. Background The majority of thousands of oocytes in the ovaries are small, non-growing and reside in preantral follicles (PFs). The development of a culture system for preantral follicle may be very useful for understanding the complex mechanism in folliculogenesis at early stages of development as wells as could offer a significant way for the propagation of livestock, including goats. The in vitro follicular culture aims to mimic what happens with a few pre- ovulatory follicles, which escapes from atresia and ovulate. For this reason, this technique is also known as “artificial ovary”. This abstract describes the results of a number of studies aimed to evaluate the effects of several substances on in vitro culture of caprine preantral follicles highlighting the many advances, limitations and prospects. Review Caprine PFs are usually cultured either in ovarian cortical slices or after isolation. Although IVC (in vitro culture) of PFs enclosed in cortical slices is practical, non-time-consuming, maintains three dimensional follicle architecture and preserves interactions between follicles and surrounding stroma cells, the cortical tissue may act as a barrier to IVC medium perfusion. Conversely, IVC of isolated PFs allows monitoring of individual follicles throughout the growing period, but is time-consuming, may be affected by the isolation procedure, demands a more sophisticated IVC system and is often applied to secondary and not to primordial and primary follicles.