Cryopreservation, and Fertility
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CRYOPRESERVATION 2021 Research and Resources ORIP’S MISSION ORIP Advances the NIH Mission by Supporting Orip.Nih.Gov Infrastructure for Innovation
OFFICE OF RESEARCH INFRASTRUCTURE PROGRAMS CRYOPRESERVATION 2021 Research and Resources ORIP’S MISSION ORIP advances the NIH mission by supporting orip.nih.gov infrastructure for innovation. This support is focused on research resources, including animal twitter.com/NIH_ORIP models for human diseases, cutting-edge scientific instrumentation, construction and modernization of research facilities, and research training Program Contacts: opportunities for veterinary scientists. Through Miguel Contreras, Ph.D. Desiree von Kollmar continued engagement with NIH Institutes, [email protected] [email protected] Centers, and Offices and the biomedical research Phone: 301-435-0045 Phone: 301-594-9410 community, ORIP empowers and expands existing Oleg Mirochnitchenko, Ph.D. Sige Zou, Ph.D. programs and develops new initiatives to support [email protected] [email protected] NIH research at the forefront of scientific progress. Phone: 301-435-0748 Phone: 301-435-0749 projects on developing efficient OVERVIEW germplasm preservation methods, including cryopreservation, for diverse Animal models are essential to understanding diseases and animal models. These projects maintaining health of humans through development of are improving existing methods diagnostic approaches and therapeutic interventions. These to preserve embryos (including disease models are being generated at an unprecedented NHP embryos), eggs, or sperm rate due to rapidly evolving technological advancements, for a variety of animal model such as gene editing. This rapid increase in animal models, species or are developing new however, is creating challenges in how to maintain these preservation methods, such critical resources in reliable and cost-effective ways. Long- as for zebrafish or Drosophila term preservation of the genetic stock of such models is melanogaster embryos. -

Molecular Beacon Nanosensors for Live Cell Detection and Tracking Differentiation and Reprogramming
Downloaded from orbit.dtu.dk on: Oct 03, 2021 Molecular beacon nanosensors for live cell detection and tracking differentiation and reprogramming Ilieva, Mirolyuba Publication date: 2013 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Ilieva, M. (2013). Molecular beacon nanosensors for live cell detection and tracking differentiation and reprogramming. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Molecular beacon nanosensors for live cell detection and tracking differentiation and reprogramming Mirolyuba Ilieva PhD Thesis April 2013 Mirolyuba Simeonova Ilieva Molecular beacon nanosensors for live cell detection and tracking differentiation and reprogramming PhD Thesis April 2013 Supervisor Assoc. Prof. Martin Dufva Co-supervisor Professor Jenny Emnéus Department of Micro- and Nanotechnology Technical University of Denmark Abstract High-sensitive and high-affinity methods to measure gene expression inside living cells have proven to be invaluable in regards to understanding fundamental processes such as cell differentiation, reprogramming, regeneration and cancer genesis. -

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

Cryopreservation of Mouse Resources Toru Takeo1* , Satohiro Nakao1, Yoshiko Nakagawa1, Jorge M
Takeo et al. Laboratory Animal Research (2020) 36:33 Laboratory Animal Research https://doi.org/10.1186/s42826-020-00066-w REVIEW Open Access Cryopreservation of mouse resources Toru Takeo1* , Satohiro Nakao1, Yoshiko Nakagawa1, Jorge M. Sztein1 and Naomi Nakagata2 Abstract The cryopreservation of sperm and embryos is useful to efficiently archive valuable resources of genetically engineered mice. Till date, more than 60,000 strains of genetically engineered mice have been archived in mouse banks worldwide. Researchers can request for the archived mouse strains for their research projects. The research infrastructure of mouse banks improves the availability of mouse resources, the productivity of research projects, and the reproducibility of animal experiments. Our research team manages the mouse bank at the Center for Animal Resources and Development in Kumamoto University and continuously develops new techniques in mouse reproductive technology to efficiently improve the system of mouse banking. In this review, we introduce the activities of mouse banks and the latest techniques used in mouse reproductive technology. Keywords: Genetically engineered mice, Mouse bank, Reproductive technology, Sperm, Embryo, Cryopreservation, In vitro fertilization, Cold storage, Hands-on workshop Introduction Resource Database (CARD R-BASE, http://cardb.cc.ku- A genetically engineered mouse is a powerful tool to eluci- mamoto-u.ac.jp/transgenic/). Till date, the international date the complex communications between genes or organs collaboration of mouse banks (International Mouse in health and diseases [1]. Moreover, humanized mouse Strain Resource: IMSR) has successfully collected more models derived from immunosuppressed mice are helpful than 60,000 strains of genetically engineered mice to bridge the gap of discovery and the development of new (Table 1). -

Educational Objectives Current IVF Practice
2/23/2015 Update from the IVF Lab: Preimplantation Genetic Testing Janet McLaren Bouknight, MD Reproductive Endocrinology and Infertility Department of Obstetrics & Gynecology Educational Objectives • Gain familiarity with the procedures and pregnancy outcomes of current in vitro fertilization practices. • Understand the application of Preimplantation Genetic Diagnosis (PGD) for single‐gene disorders. • Appreciate the role of Preimplantation Genetic Screening (PGS) in maximizing IVF cycle efficiency and encouraging elective single embryo transfer. Current IVF Practice 1 2/23/2015 Current IVF Practice 150,000 cycles 65,000 livebirths Current IVF Practice Current IVF Practice All SART Clinics 2012 <35 35‐37 38‐40 41‐42 >42 Pregnancy Rate 47% 38% 30% 20% 9% Live Birth Rate 41% 31% 22% 12% 4% # embryos transfer 1.9 2.0 2.4 2.9 2.9 % Twins 30% 25% 20% 13% 9% • What are the advanced that have contributed to an increase in success in the IVF Lab? • Improved lab conditions • Extended embryo culture • Improvements in embryo cryopreservation 2 2/23/2015 Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions 3 2/23/2015 Current IVF Practice: Extended Embryo Culture Day 3: Cleavage Stage Day 5: Blastocyst Stage • 8‐cells • 200‐300 cells • 30% implantation rate • 50% implantation rate Increased live birth rates seen in “good prognosis” patients who undergo a Day 5 transfer Current IVF Practice: Extended Embryo Culture Current IVF Practice: Extended Embryo Culture • Supports blastocyst transfer for “good prognosis” patients • Opportunity to consider single embryo transfer • May be associated with a small increased risk of monozygotic twinning 4 2/23/2015 Current IVF Practice: Embryo Cryopreservation Cycles • Improvements in freezing techniques (vitrification) have increased success of cryopreserved embryos. -
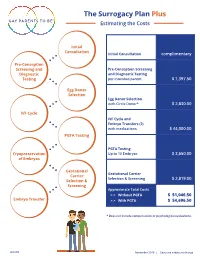
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

CRYOPRESERVATION Cryoprotectant Chemicals an Integral Part of Cell Culture Involves the Successful Preservation of Im- Portant Cell Lines
CRYOPRESERVATION Cryoprotectant Chemicals An integral part of cell culture involves the successful preservation of im- portant cell lines. A primary factor for success is developing an appropri- DIMETHYL SULFOXIDE 25 ml 8.90 196055 100 ml 15.40 ate protocol for a particular cell line. A common misconception among RT [67-68-5] (DMSO) 500 ml 27.40 scientists is that preserving methods for a given cell type are transfer- Cell Culture Reagent 1 liter 45.60 able to similar cell types. Although, this may be true on occassion, it is Purity: ≥99% far from the general rule. Protocols require modification for each cell Ideal for use as a cryoprotectant. NOT FOR HUMAN USE! type. Another key factor for successful preservation is the use of an ef- C2H6SO MW 78.13 fective cryoprotectant. The discovery of glycerol as a preservation me- ETHYLENE GLYCOL 250 ml 10.80 dium led to the rapid discovery of other protectants such as methanol, 151089 1 liter 22.50 RT [107-21-1] ethylene glycol, and DMSO (dimethyl sulfoxide). Purity: 99% min. 1 ml = approx. 1.11 g With any cryopreservation process, the primary objective is maximum C2H6O2 MW 62.1 recovery with minimal damage to the cells. The absence of an effective D-(+)-GLUCOSE 100 g 15.00 cryoprotectant leads to the formation of intracellular ice crystals which 194672 1 kg 23.50 RT [50-99-7] puncture organelles and cell membranes. Also, as water within the me- (Dextrose; Corn sugar) 5 kg 94.00 dium freezes, cells become dehydrated altering the salt concentration Cell Culture Reagent Anhydrous and causing morphological or biological changes after recovery. -

Social Freezing: Pressing Pause on Fertility
International Journal of Environmental Research and Public Health Review Social Freezing: Pressing Pause on Fertility Valentin Nicolae Varlas 1,2 , Roxana Georgiana Bors 1,2, Dragos Albu 1,2, Ovidiu Nicolae Penes 3,*, Bogdana Adriana Nasui 4,* , Claudia Mehedintu 5 and Anca Lucia Pop 6 1 Department of Obstetrics and Gynaecology, Filantropia Clinical Hospital, 011171 Bucharest, Romania; [email protected] (V.N.V.); [email protected] (R.G.B.); [email protected] (D.A.) 2 Department of Obstetrics and Gynaecology, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 3 Department of Intensive Care, University Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 4 Department of Community Health, “Iuliu Hat, ieganu” University of Medicine and Pharmacy, 6 Louis Pasteur Street, 400349 Cluj-Napoca, Romania 5 Department of Obstetrics and Gynaecology, Nicolae Malaxa Clinical Hospital, 020346 Bucharest, Romania; [email protected] 6 Department of Clinical Laboratory, Food Safety, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia Street, 020945 Bucharest, Romania; [email protected] * Correspondence: [email protected] (O.N.P.); [email protected] (B.A.N.) Abstract: Increasing numbers of women are undergoing oocyte or tissue cryopreservation for medical or social reasons to increase their chances of having genetic children. Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. The purpose of this study was to summarize recent findings focusing on the challenges of elective egg freezing. -

Effects of Sample Treatments on Genome Recovery Via Single-Cell Genomics
The ISME Journal (2014) 8, 2546–2549 & 2014 International Society for Microbial Ecology All rights reserved 1751-7362/14 www.nature.com/ismej SHORT COMMUNICATION Effects of sample treatments on genome recovery via single-cell genomics Scott Clingenpeel1, Patrick Schwientek1, Philip Hugenholtz2 and Tanja Woyke1 1DOE Joint Genome Institute, Walnut Creek, CA, USA and 2Australian Centre for Ecogenomics, School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, Queensland, Australia Single-cell genomics is a powerful tool for accessing genetic information from uncultivated microorganisms. Methods of handling samples before single-cell genomic amplification may affect the quality of the genomes obtained. Using three bacterial strains we show that, compared to cryopreservation, lower-quality single-cell genomes are recovered when the sample is preserved in ethanol or if the sample undergoes fluorescence in situ hybridization, while sample preservation in paraformaldehyde renders it completely unsuitable for sequencing. The ISME Journal (2014) 8, 2546–2549; doi:10.1038/ismej.2014.92; published online 13 June 2014 Relatively little is known about the functioning of genomes from single cells. Three methods of sample complex microbial communities, largely due to the preservation were tested: cryopreservation with difficulty in culturing most microbes (Rappe and 20% glycerol as a cryoprotectant, preservation in Giovannoni, 2003). Although metagenomics can 70% ethanol, and preservation in 4% paraformalde- provide information on the genetic capabilities of hyde. Because paraformaldehyde treatment causes the entire community, it is difficult to connect crosslinks between nucleic acids and proteins, we predicted gene functions to specific organisms using tested cells exposed to 4% paraformaldehyde with metagenomics (Morales and Holben, 2011). -

Approaches and Technologies in Male Fertility Preservation
International Journal of Molecular Sciences Review Approaches and Technologies in Male Fertility Preservation Mahmoud Huleihel 1,2,* and Eitan Lunenfeld 2,3,4 1 The Shraga Segal Department of Microbiology, Immunology and Genetics, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel 2 The Center of Advanced Research and Education in Reproduction (CARER), Ben-Gurion University of the Negev, Beer Sheva 84105, Israel; lunenfl[email protected] 3 Department of OB/GYN, Soroka Medical Center, Beer Sheva 8410501, Israel 4 Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel * Correspondence: [email protected]; Tel.: +972-86479959 Received: 28 May 2020; Accepted: 29 July 2020; Published: 31 July 2020 Abstract: Male fertility preservation is required when treatment with an aggressive chemo-/-radiotherapy, which may lead to irreversible sterility. Due to new and efficient protocols of cancer treatments, surviving rates are more than 80%. Thus, these patients are looking forward to family life and fathering their own biological children after treatments. Whereas adult men can cryopreserve their sperm for future use in assistance reproductive technologies (ART), this is not an option in prepubertal boys who cannot produce sperm at this age. In this review, we summarize the different technologies for male fertility preservation with emphasize on prepubertal, which have already been examined and/or demonstrated in vivo and/or in vitro using animal models and, in some cases, using human tissues. We discuss the limitation of these technologies for use in human fertility preservation. This update review can assist physicians and patients who are scheduled for aggressive chemo-/radiotherapy, specifically prepubertal males and their parents who need to know about the risks of the treatment on their future fertility and the possible present option of fertility preservation.