MG Infertility Services Commercial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Rapid and Effective Nonsurgical Artificial Insemination Protocol Using
Transgenic Res (2015) 24:775–781 DOI 10.1007/s11248-015-9887-3 TECHNICAL REPORT A rapid and effective nonsurgical artificial insemination protocol using the NSETTM device for sperm transfer in mice without anesthesia Barbara J. Stone . Kendra H. Steele . Angelika Fath-Goodin Received: 27 January 2015 / Accepted: 3 June 2015 / Published online: 12 June 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Artificial insemination (AI) is an assisted Introduction reproductive technique that is implemented success- fully in humans as a fertility treatment, performed Artificial insemination (AI) is used to assist in extensively for commercial breeding of livestock, and reproduction by directly delivering motile sperm to is also successful in laboratory rodents. AI in the the female reproductive tract. Artificial insemination mouse may be especially useful for breeding of in mice can be useful as an alternative to breeding for transgenic or mutant mice with fertility problems, strains which are not easily bred due to physical or expansion of mouse colonies, and as an alternative to behavioral issues. The techniques of AI and in vitro in vitro fertilization. Nonsurgical AI techniques for the fertilization (IVF) can both be used to generate viable mouse have been described previously but are not embryos. Development after AI simply continues in often implemented due to technical difficulties. Here the recipient female, while embryos generated from we compare various protocols for preparation of CD1 IVF must be transferred to a pseudopregnant recipient. recipients prior to AI for naı¨ve (in estrus), ovulation- AI or IVF can be used to generate embryos after induced, and superovulated females. -

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

The Artificial Way
SPOTLIGHT Breeding Rhinos for the future The Artificial way BY FELIX PATTON aptive breeding programmes have many decades about the husbandry and had some spectacular successes in reproductive needs of captive Black and 310 Csaving species and reintroducing Indian rhinos, when applied to White them into the wild. All rhino species Number of oestrous cycles rhinos, it has been unsuccessful. The are at risk from poaching, disease and non-reproducing White rhino main problem has been the absence of or natural disaster. Developing captive female in captivity can exhibit. erratic nature of oestrous cycle activity in populations is an essential safeguard to over half of the females in the European avoid extinction but breeding success has and North American Species Survival been limited. If a female rhino does not Program. get pregnant, her uterus starts to develop White rhino females in the wild irreversible problems, such as cysts To date, captive reproduction in usually experience short intervals and tumours. Can the failure to become the White rhino has been poor. Despite between successive births, even as pregnant be overcome? the wealth of knowledge acquired over little as 18 months. This indicates that pregnancy and lactation are the most common endocrine profile with possibly as few as 30 oestrous cycles per reproductive life span. Ultrasound technology for use in rhinoceros has been A reproducing female White rhino in used in well over 150 reproductive assessments in more captivity may produce up to nine calves. With a pregnancy of 16 months and than 70 rhinos with the result that the causes of poor subsequent lactation of approximately captive performance are now known. -

Oocyte Or Embryo Donation to Women of Advanced Reproductive Age: an Ethics Committee Opinion
ASRM PAGES Oocyte or embryo donation to women of advanced reproductive age: an Ethics Committee opinion Ethics Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Advanced reproductive age (ARA) is a risk factor for female infertility, pregnancy loss, fetal anomalies, stillbirth, and obstetric com- plications. Oocyte donation reverses the age-related decline in implantation and birth rates of women in their 40s and 50s and restores pregnancy potential beyond menopause. However, obstetrical complications in older patients remain high, particularly related to oper- ative delivery and hypertensive and cardiovascular risks. Physicians should perform a thorough medical evaluation designed to assess the physical fitness of a patient for pregnancy before deciding to attempt transfer of embryos to any woman of advanced reproductive age (>45 years). Embryo transfer should be strongly discouraged or denied to women of ARA with underlying conditions that increase or exacerbate obstetrical risks. Because of concerns related to the high-risk nature of pregnancy, as well as longevity, treatment of women over the age of 55 should generally be discouraged. This statement replaces the earlier ASRM Ethics Committee document of the same name, last published in 2013 (Fertil Steril 2013;100:337–40). (Fertil SterilÒ 2016;106:e3–7. Ó2016 by American Society for Reproductive Medicine.) Key Words: Ethics, third-party reproduction, complications, pregnancy, parenting Discuss: You can discuss -
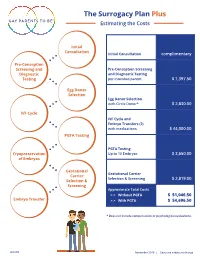
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Artificial Insemination
Ch36-A03309.qxd 1/23/07 5:16 PM Page 539 Section 6 Infertility and Recurrent Pregnancy Loss Chapter Artificial Insemination 36 Ashok Agarwal and Shyam S. R. Allamaneni INTRODUCTION widely available, the terms homologous artificial insemination and heterologous artificial insemination were used to differentiate Artificial insemination is an assisted conception method that can these two alternative sources. However, the use of these bio- be used to alleviate infertility in selected couples. The rationale medical terms in this manner is at variance with their scientific behind the use of artificial insemination is to increase the gamete meaning, where they denote different species or organisms (as in, density near the site of fertilization.1 The effectiveness of artificial e.g., homologous and heterologous tissue grafts). insemination has been clearly established in specific subsets of In the latter half of the 20th century, the terms artificial infertile patients such as those with idiopathic infertility, infertility insemination, donor (AID) and artificial insemination, husband related to a cervical factor, or a mild male factor infertility (AIH) found common use. However, the widespread use of the (Table 36-1).2,3 An accepted advantage of artificial insemination acronym AIDS for acquired immunodeficiency syndrome resulted is that it is generally less expensive and invasive than other in the replacement of AID with therapeutic donor insemination assisted reproductive technology (ART) procedures.4 (TDI). An analogous alternative term for AIH has not evolved, This chapter provides a comprehensive description of probably in part because of the increasingly common situation indications for artificial insemination, issues to consider before where the woman’s partner is not her legal husband. -

In Vitro Fertilization and Artificial Insemination: Ethical Consideration
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1997 In Vitro Fertilization and Artificial Insemination: Ethical Consideration Joseph Ibegbulem Ekweariri Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Philosophy Commons Recommended Citation Ekweariri, Joseph Ibegbulem, "In Vitro Fertilization and Artificial Insemination: Ethical Consideration" (1997). Dissertations. 3716. https://ecommons.luc.edu/luc_diss/3716 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1997 Joseph Ibegbulem Ekweariri LOYOLA UNIVERSITY OF CHICAGO IN VITRO FERTILIZATION AND ARTIFICIAL INSEMINATION: ETHICAL CONSIDERATION A DISSERTATION SUBMITTED TO THEFACULTYOFTHEGRADUATESCHOOL IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF PHILOSOPHY BY JOSEPH IBEGBULEM EKWEARIRI CHICAGO, ILLINOIS MAY, 1997 Copyright by Joseph Ibegbulem Ekweariri, 1997 All rights reserved. ACKNOWLEDGMENTS I thank you God for my life; even when ill-health threatened my studies and this work you sustained me throughout. Many people contributed to the success of this work in different capacities. I wish to thank them. I am grateful to my dissertation committee: Prof. David T. Ozar, the Chairman of the committee and director of my dissertation, Prof. Richard Westley, and Prof. John Langan S.J. I wish to thank all my professors at Loyola University, especially Prof. Kenneth Thompson, the director of the ~raduate School of Philosophy and Max Caproni Asst. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

The Protection of the Human Embryo in Vitro
Strasbourg, 19 June 2003 CDBI-CO-GT3 (2003) 13 STEERING COMMITTEE ON BIOETHICS (CDBI) THE PROTECTION OF THE HUMAN EMBRYO IN VITRO Report by the Working Party on the Protection of the Human Embryo and Fetus (CDBI-CO-GT3) Table of contents I. General introduction on the context and objectives of the report ............................................... 3 II. General concepts............................................................................................................................... 4 A. Biology of development ....................................................................................................................... 4 B. Philosophical views on the “nature” and status of the embryo............................................................ 4 C. The protection of the embryo............................................................................................................... 8 D. Commercialisation of the embryo and its parts ................................................................................... 9 E. The destiny of the embryo ................................................................................................................... 9 F. “Freedom of procreation” and instrumentalisation of women............................................................10 III. In vitro fertilisation (IVF).................................................................................................................. 12 A. Presentation of the procedure ...........................................................................................................12 -

Social Freezing: Pressing Pause on Fertility
International Journal of Environmental Research and Public Health Review Social Freezing: Pressing Pause on Fertility Valentin Nicolae Varlas 1,2 , Roxana Georgiana Bors 1,2, Dragos Albu 1,2, Ovidiu Nicolae Penes 3,*, Bogdana Adriana Nasui 4,* , Claudia Mehedintu 5 and Anca Lucia Pop 6 1 Department of Obstetrics and Gynaecology, Filantropia Clinical Hospital, 011171 Bucharest, Romania; [email protected] (V.N.V.); [email protected] (R.G.B.); [email protected] (D.A.) 2 Department of Obstetrics and Gynaecology, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 3 Department of Intensive Care, University Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 4 Department of Community Health, “Iuliu Hat, ieganu” University of Medicine and Pharmacy, 6 Louis Pasteur Street, 400349 Cluj-Napoca, Romania 5 Department of Obstetrics and Gynaecology, Nicolae Malaxa Clinical Hospital, 020346 Bucharest, Romania; [email protected] 6 Department of Clinical Laboratory, Food Safety, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia Street, 020945 Bucharest, Romania; [email protected] * Correspondence: [email protected] (O.N.P.); [email protected] (B.A.N.) Abstract: Increasing numbers of women are undergoing oocyte or tissue cryopreservation for medical or social reasons to increase their chances of having genetic children. Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. The purpose of this study was to summarize recent findings focusing on the challenges of elective egg freezing. -

Assisted Reproduction in Developing Countries—Facing up to the Issues
1 Progress, No. 63 No. 63 2003 UNDP/UNFPA/WHO/World Assisted reproduction in developing Bank Special Programme of Research, Development and countriesfacing up to the issues Research Training in Human Reproduction (HRP) For millions of couples around the Attitudes to assisted reproduction are Department of Reproductive world, the inability to have children is very much influenced by the specific Health and Research, a personal tragedy. For a significant cultural and social context. Manipula- World Health Organization, proportion of them, the private agony tion of sperm, oocytes and embryos Geneva, Switzerland is compounded by a social stigma, raises numerous questions to which which can have serious and far-reach- there are no easy answers, and which Launched by the World Health ing consequences. It is not surprising often arouse strong emotions. The Organization in 1972, the UNDP/ therefore that the demand for as- article on page 5 outlines some of the UNFPA/WHO/World Bank sisted reproductive technologies ethical and legal issues surrounding Special Programme of (ART) is growing in all regions. But the use of ART, which need to be Research, Development and how can the provision of these tech- discussed openly by all involved par- Research Training in Human nologieswhich are expensive and ties wherever such services are made Reproduction is a global have a success rate of less than available. programme of technical 30%be justified in developing coun- cooperation. It promotes, tries with poorly developed health In the 25 years since the birth of the coordinates, supports, conducts, services that are still struggling with first baby resulting from in vitro ferti- and evaluates research on infectious and chronic diseases, such lization, there have been tremendous reproductive health, with as tuberculosis, malaria and HIV/ advances in scientific techniques and particular reference to the needs AIDS? medical applications of ART.