Report of Four Donor-Recipient Oocyte Cryopreservation Cycles Resulting in High Pregnancy and Implantation Rates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

Oocyte Or Embryo Donation to Women of Advanced Reproductive Age: an Ethics Committee Opinion
ASRM PAGES Oocyte or embryo donation to women of advanced reproductive age: an Ethics Committee opinion Ethics Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Advanced reproductive age (ARA) is a risk factor for female infertility, pregnancy loss, fetal anomalies, stillbirth, and obstetric com- plications. Oocyte donation reverses the age-related decline in implantation and birth rates of women in their 40s and 50s and restores pregnancy potential beyond menopause. However, obstetrical complications in older patients remain high, particularly related to oper- ative delivery and hypertensive and cardiovascular risks. Physicians should perform a thorough medical evaluation designed to assess the physical fitness of a patient for pregnancy before deciding to attempt transfer of embryos to any woman of advanced reproductive age (>45 years). Embryo transfer should be strongly discouraged or denied to women of ARA with underlying conditions that increase or exacerbate obstetrical risks. Because of concerns related to the high-risk nature of pregnancy, as well as longevity, treatment of women over the age of 55 should generally be discouraged. This statement replaces the earlier ASRM Ethics Committee document of the same name, last published in 2013 (Fertil Steril 2013;100:337–40). (Fertil SterilÒ 2016;106:e3–7. Ó2016 by American Society for Reproductive Medicine.) Key Words: Ethics, third-party reproduction, complications, pregnancy, parenting Discuss: You can discuss -
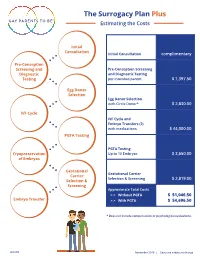
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

Social Freezing: Pressing Pause on Fertility
International Journal of Environmental Research and Public Health Review Social Freezing: Pressing Pause on Fertility Valentin Nicolae Varlas 1,2 , Roxana Georgiana Bors 1,2, Dragos Albu 1,2, Ovidiu Nicolae Penes 3,*, Bogdana Adriana Nasui 4,* , Claudia Mehedintu 5 and Anca Lucia Pop 6 1 Department of Obstetrics and Gynaecology, Filantropia Clinical Hospital, 011171 Bucharest, Romania; [email protected] (V.N.V.); [email protected] (R.G.B.); [email protected] (D.A.) 2 Department of Obstetrics and Gynaecology, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 3 Department of Intensive Care, University Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 4 Department of Community Health, “Iuliu Hat, ieganu” University of Medicine and Pharmacy, 6 Louis Pasteur Street, 400349 Cluj-Napoca, Romania 5 Department of Obstetrics and Gynaecology, Nicolae Malaxa Clinical Hospital, 020346 Bucharest, Romania; [email protected] 6 Department of Clinical Laboratory, Food Safety, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia Street, 020945 Bucharest, Romania; [email protected] * Correspondence: [email protected] (O.N.P.); [email protected] (B.A.N.) Abstract: Increasing numbers of women are undergoing oocyte or tissue cryopreservation for medical or social reasons to increase their chances of having genetic children. Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. The purpose of this study was to summarize recent findings focusing on the challenges of elective egg freezing. -

The Disposition of Cryopreserved Embryos: Why Embryo Adoption Is an Inapposite Model for Application to Third-Party Assisted Reproduction Elizabeth E
William Mitchell Law Review Volume 35 | Issue 2 Article 1 2009 The Disposition of Cryopreserved Embryos: Why Embryo Adoption Is an Inapposite Model for Application to Third-Party Assisted Reproduction Elizabeth E. Swire Falker Follow this and additional works at: http://open.mitchellhamline.edu/wmlr Recommended Citation Falker, Elizabeth E. Swire (2009) "The Disposition of Cryopreserved Embryos: Why Embryo Adoption Is an Inapposite Model for Application to Third-Party Assisted Reproduction," William Mitchell Law Review: Vol. 35: Iss. 2, Article 1. Available at: http://open.mitchellhamline.edu/wmlr/vol35/iss2/1 This Article is brought to you for free and open access by the Law Reviews and Journals at Mitchell Hamline Open Access. It has been accepted for inclusion in William Mitchell Law Review by an authorized administrator of Mitchell Hamline Open Access. For more information, please contact [email protected]. © Mitchell Hamline School of Law Falker: The Disposition of Cryopreserved Embryos: Why Embryo Adoption Is THE DISPOSITION OF CRYOPRESERVED EMBRYOS: WHY EMBRYO ADOPTION IS AN INAPPOSITE MODEL FOR APPLICATION TO THIRD-PARTY ASSISTED REPRODUCTION † Elizabeth E. Swire Falker, Esq. I. THE DEBATE OVER THE DISPOSITION OF CRYOPRESERVED EMBRYOS FOR FAMILY BUILDING ............................................ 491 II. THE “TYPICAL” EMBRYO ADOPTION/DONATION IN THE UNITED STATES ...................................................................... 498 III. DEFINING THE TERM EMBRYO ............................................... -

Should the U.S. Approve Mitochondrial Replacement Therapy?
SHOULD THE U.S. APPROVE MITOCHONDRIAL REPLACEMENT THERAPY? An Interactive Qualifying Project Report Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE In partial fulfillment of the requirements for the Degree of Bachelor of Science By: ____________________ ____________________ ____________________ Daniela Barbery Emily Caron Daniel Eckler IQP-43-DSA-6594 IQP-43-DSA-7057 IQP-43-DSA-5020 ____________________ ____________________ Benjamin Grondin Maureen Hester IQP-43-DSA-5487 IQP-43-DSA-2887 August 27, 2015 APPROVED: _________________________ Prof. David S. Adams, PhD WPI Project Advisor 1 ABSTRACT The overall goal of this project was to document and evaluate the new technology of mitochondrial replacement therapy (MRT), and to assess its technical, ethical, and legal problems to help determine whether MRT should be approved in the U.S. We performed a review of the current research literature and conducted interviews with academic researchers, in vitro fertility experts, and bioethicists. Based on the research performed for this project, our team’s overall recommendation is that the FDA approve MRT initially for a small number of patients, and follow their offspring’s progress closely for a few years before allowing the procedure to be done on a large scale. We recommend the FDA approve MRT only for treating mitochondrial disease, and recommend assigning parental rights only to the two nuclear donors. In medical research, animal models are useful but imperfect, and in vitro cell studies cannot provide information on long-term side-effects, so sometimes we just need to move forward with closely monitored human experiments. 2 TABLE OF CONTENTS Title Page ……………………………….……………………………………..……. 01 Abstract …………………………………………………………………..…………. 02 Table of Contents ………………………………………………………………..… 03 Acknowledgements …………………………………………………………..……. -

Donor Eggs for the Treatment of Infertility
CLINICAL Caitlin Dunne, MD, FRCSC Donor eggs for the treatment of infertility Using donated eggs can be a remarkably successful fertility treatment in the right circumstances. Though donor egg pregnancies may carry some increased obstetrical risks, the risks are manageable and can offer women a chance at pregnancy when there is no other option. ABSTRACT: Egg donation is a common treatment the right time to recover the resulting embryo, Columbia has the highest age of first birth in for infertility. It is most often used for women with which was transferred into the intended moth- Canada at 30.5 years versus 30.3 years in On- premature menopause, advanced reproductive er’s uterus.2,3 tario.10 According to Statistics Canada, 2010 age, or a history of unsuccessful in vitro fertilization In the early 1980s, assisted reproductive marked the first time that more women in their attempts. Because egg donors are generally in their technology was developing rapidly in Canada 30s were having children compared to women 20s, pregnancy success rates are high. In many cas- and around the world.4 Fertility pioneers used in their 20s.11 es, donor eggs give women a chance at pregnancy laparoscopy to retrieve donor eggs for fertiliza- The possible consequences of delaying child- when there would be no other option. Donor egg tion in vitro.3,5,6 These early “third-party repro- bearing are infertility, embryo aneuploidy, and pregnancies may carry some increased obstetrical duction” techniques were groundbreaking at miscarriage. These are largely attributed to aging risks related to preeclampsia and advanced mater- the time, given that the world’s first IVF baby, oocytes with failing meiotic spindles and other nal age. -

Let She Who Has the Womb Speak: Regulating the Use of Human Oocyte Cryopreservation to the Detriment of Older Women
Arkansas Law Review Volume 72 Number 3 Article 2 February 2020 Let She Who has the Womb Speak: Regulating the use of Human Oocyte Cryopreservation to the Detriment of Older Women Browne C. Lewis Cleveland State University Follow this and additional works at: https://scholarworks.uark.edu/alr Part of the Family Law Commons Recommended Citation Browne C. Lewis, Let She Who has the Womb Speak: Regulating the use of Human Oocyte Cryopreservation to the Detriment of Older Women, 72 Ark. L. Rev. 597 (2020). Available at: https://scholarworks.uark.edu/alr/vol72/iss3/2 This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Arkansas Law Review by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. LET SHE WHO HAS THE WOMB SPEAK: REGULATING THE USE OF HUMAN OOCYTE CRYOPRESERVATION TO THE DETRIMENT OF OLDER WOMEN Browne C. Lewis INTRODUCTION “Inequality starts in the womb.”1 When it comes to childbearing, advances in assisted reproductive technology (ART) may negate the veracity of this quote. In her autobiography Becoming,2 former First Lady Michelle Obama discusses her struggles with infertility.3 Mrs. Obama’s difficulty getting pregnant may have stemmed from the fact that she postponed motherhood to focus on her career as a high-powered attorney.4 At that time, for Mrs. Obama and women of her generation, the focus was on pregnancy prevention instead of procreation preservation.5 Women feared being placed on the “mommy track,”6 so they waited to have children until after they had achieved success in their careers.7 Some of those women paid B.A., Grambling State University, M.P.P., Humphrey Institute; J.D., University of Minnesota School of Law; University of Houston Law Center. -

Specific Protocols of Controlled Ovarian Stimulation for Oocyte Cryopreservation in Breast Cancer Patients
OVARIAN STIMULATION IN BREAST CANCERORIGINAL PATIENTS, CavagnaARTICLE et al. Specific protocols of controlled ovarian stimulation for oocyte cryopreservation in breast cancer patients † F. Cavagna MD,* A. Pontes MD, M. Cavagna MD,* A. Dzik MD,* N.F. Donadio MD,* R. Portela BSc,* M.T. Nagai MD,* and L.H. Gebrim MD* ABSTRACT Background Fertility preservation is an important concern in breast cancer patients. In the present investigation, we set out to create a specific protocol of controlled ovarian stimulation (cos) for oocyte cryopreservation in breast cancer patients. Methods From November 2014 to December 2016, 109 patients were studied. The patients were assigned to a specific random-start ovarian stimulation protocol for oocyte cryopreservation. The endpoints were the numbers of oocytes retrieved and of mature oocytes cryopreserved, the total number of days of ovarian stimulation, the total dose of gonadotropin administered, and the estradiol level on the day of the trigger. Results Mean age in this cohort was 31.27 ± 4.23 years. The average duration of cos was 10.0 ± 1.39 days. The mean number of oocytes collected was 11.62 ± 7.96 and the mean number of vitrified oocytes was 9.60 ± 6.87. The mean estradiol concentration on triggering day was 706.30 ± 450.48 pg/mL, and the mean dose of gonadotropins administered was 2610.00 ± 716.51 IU. When comparing outcomes by phase of the cycle in which cos was commenced, we observed no significant differences in the numbers of oocytes collected and vitrified, the length of ovarian stimulation, and the estradiol level on trigger day. -

Science of Mitochondrial Donation and Related Matters Submission 20
+- Associate Professor Catherine Mills Monash Bioethics Centre Monash University [email protected] +61 3 9905 3207 Committee Secretary Senate Standing Committees on Community Affairs PO Box 6100 Parliament House Canberra ACT 2600 11 May 2018 Dear Committee Members. Thank you for the opportunity to contribute to the Inquiry into the Science of Mitochondrial Donation and Other Matters. We are the Chief Investigators of an ongoing Australian Research Council-funded study into ‘The legal and ethical aspects of the inheritable genetic modification of humans: The Australian context’ (ARC DP170100919). This project focuses on the ethical and legal aspects of mitochondrial donation and CRISPR-Cas9. We hope our research to date assists the Committee in considering the legal and ethical aspects of mitochondrial donation and associated matters. Sincerely, Associate Professor Catherine Mills Lead Investigator Monash Bioethics Centre Monash University Associate Professor Karinne Ludlow Faculty of Law Monash University Professor Robert Sparrow Department of Philosophy Monash University Dr Narelle Warren School of Social Sciences Monash University INQUIRY INTO THE SCIENCE OF MITOCHONDRIAL DONATION AND OTHER MATTERS | 1 OVERVIEW Mitochondrial donation is a relatively new scientific advance in the sphere of assisted reproductive technologies. It allows for the replacement of mitochondrial DNA affected by mutations in a human egg (ovum) or zygote by transferring the nuclear DNA into a healthy donated egg that is not affected. The aim of this is to stop the transmission of mitochondrial disease that is inherited from the maternal line. In 2015, the British Parliament legalised the clinical use of mitochondrial donation. Significantly, these techniques allow for interventions that may be inherited by all subsequent generations of offspring of the person that the modified embryo may grow to be.