The Recurrent Laryngeal Cardiac Nerve in Fetuses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association of Arrhythmia in Patients with Cervical Spondylosis: a Nationwide Population-Based Cohort Study
Journal of Clinical Medicine Article Association of Arrhythmia in Patients with Cervical Spondylosis: A Nationwide Population-Based Cohort Study Shih-Yi Lin 1,2, Wu-Huei Hsu 1,3, Cheng-Chieh Lin 1,4, Cheng-Li Lin 5,6, Chun-Hao Tsai 1,7, Chih-Hsueh Lin 1,4, Der-Cherng Chen 7, Tsung-Chih Lin 8, Chung-Y. Hsu 1 and Chia-Hung Kao 1,9,10,* ID 1 Graduate Institute of Biomedical Sciences and School of Medicine, College of Medicine, China Medical University, No. 2, Yuh-Der Road, Taichung 404, Taiwan; [email protected] (S.-Y.L.); [email protected] (W.-H.H.); [email protected] (C.-C.L.); [email protected] (C.-H.T.); [email protected] (C.-H.L.); [email protected] (C.-Y.H.) 2 Division of Nephrology and Kidney Institute, China Medical University Hospital, Taichung 404, Taiwan 3 Division of Pulmonary and Critical Care Medicine, China Medical University Hospital and China Medical University, Taichung 404, Taiwan 4 Department of Family Medicine, China Medical University Hospital, Taichung 404, Taiwan 5 Management Office for Health Data, China Medical University Hospital, Taichung 404, Taiwan; [email protected] 6 College of Medicine, China Medical University, Taichung 404, Taiwan 7 Department of Orthopedics, China Medical University Hospital, Taichung 404, Taiwan; [email protected] 8 Department of Orthopedics, St. Martin De Porres Hospital, Chiayi 600, Taiwan; [email protected] 9 Department of Nuclear Medicine, China Medical University Hospital, Taichung 404, Taiwan 10 Department of Bioinformatics and Medical Engineering, Asia University, Taichung 413, Taiwan * Correspondence: [email protected]; Tel.: +886-4-2205-2121 (ext. -

Sympathetic Tales: Subdivisons of the Autonomic Nervous System and the Impact of Developmental Studies Uwe Ernsberger* and Hermann Rohrer
Ernsberger and Rohrer Neural Development (2018) 13:20 https://doi.org/10.1186/s13064-018-0117-6 REVIEW Open Access Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies Uwe Ernsberger* and Hermann Rohrer Abstract Remarkable progress in a range of biomedical disciplines has promoted the understanding of the cellular components of the autonomic nervous system and their differentiation during development to a critical level. Characterization of the gene expression fingerprints of individual neurons and identification of the key regulators of autonomic neuron differentiation enables us to comprehend the development of different sets of autonomic neurons. Their individual functional properties emerge as a consequence of differential gene expression initiated by the action of specific developmental regulators. In this review, we delineate the anatomical and physiological observations that led to the subdivision into sympathetic and parasympathetic domains and analyze how the recent molecular insights melt into and challenge the classical description of the autonomic nervous system. Keywords: Sympathetic, Parasympathetic, Transcription factor, Preganglionic, Postganglionic, Autonomic nervous system, Sacral, Pelvic ganglion, Heart Background interplay of nervous and hormonal control in particular The “great sympathetic”... “was the principal means of mediated by the sympathetic nervous system and the ad- bringing about the sympathies of the body”. With these renal gland in adapting the internal -

The Axatomy of the Autonomic Nervous System in the Dog1
THE AXATOMY OF THE AUTONOMIC NERVOUS SYSTEM IN THE DOG1 NICHOLAS JAMES AIIZERES Ucpartnitiit of Aiintoiiry, Cnzvemtty of Xtclizgaii Scliool of ;2/cdicmc. Ann Arbor, Mtclatgan ELEVEN FIGURES INTRODUCTTOX I<iiowledgc clerivccl from cmprimeiits on tlie clog has not infrequently been applied to man without considering dif- fcmnccs in aiiatoniy. This is c~spcciallytrue of the autonomic nervous systeni, oiily parts of which have b:mi described in the adult clog. The cardiac ncli'vcs \\'ere described by Sc1iuran.- Iew ( '27) ant1 Soniclez ( '39), the gcw~alplan of the abrloniirial ancl sacral regions by Trumble ( '34), tlic lunibosacral trunk by Alehler, Fisclier and Alexander ( '52), the vagi lsy Hilsa- beck aid Hill ( '50) and BIcC'rca and D'hrcy ( '%), ant1 the urogenital plexuses by Schal~adasch( '26) aiicl Ncdowar ( '2.3. The present study was undertaken to fill gaps in previous clescriptions ancl to present an over-all view of the autonomic iierrous system in the (log, esclutliiig the cephalic region. In the pi-c~seiitiiivcstigation the XI< terminology has been used iiistpad of the usual RNA familiar in human anatomy loccause tlic study was based on the clog. Sincc a dog is a pronograde niamnial the terms cranial aiid caudal seeni nior(t appropriat r tliaii the ESA terms superior and inferior. Tiiis paper is n condensation of a clissei t:ition snbniittctl in paytial fulfillmc.~it of tlrc rcqiiireiiieuts for the drgrce of Doctor of Pliilosopliy in the University of Rlicliigaii. I wish to tlimik Dr. It. T. Wootlhuine for Ills interest and aclriec. For the supply of nlatciial tlie author vihlies to e\-pr('\s his appreciatioir to mcmbc~is of the 1)ep:irtiiiciits of P1iTsiolog.y :iiiil PIiar~u:~cologrof tlic Viiirersity of hliclrigan. -

Neurobiology of Visceral Pain
Neurobiology of Visceral Pain Definition Pain arising from the internal organs of the body: • Heart, great vessels, and perivascular structures (e.g., lymph nodes) • Airway structures (pharynx, trachea, bronchi, lungs, pleura) • Gastrointestinal tract (esophagus, stomach, small intestine, colon, rectum) • Upper-abdominal structures (liver, gallbladder, biliary tree, pancreas, spleen) • Urological structures (kidneys, ureters, urinary bladder, urethra) • Reproductive organs (uterus, ovaries, vagina, testes, vas deferens, prostate) • Omentum, visceral peritoneum Clinical Features of Visceral Pain Key features associated with pain from the viscera include diffuse localization, an unreliable association with pathology, and referred sensations. Strong autonomic and emotional responses may be evoked with minimal sensation. Referred pain has two components: (1) a localization of the site of pain generation to somatic tissues with nociceptive processing at the same spinal segments (e.g., chest and arm pain from cardiac ischemia) and (2) a sensitization of these segmental tissues (e.g., kidney stones may cause the muscles of the lateral torso to become tender to palpation). These features are in contrast to cutaneous pain, which is well localized and features a graded stimulus-response relationship. Anatomy of Neurological Structures Pathways for visceral sensation are diffusely organized both peripherally and centrally. Primary afferent nerve fibers innervating viscera project into the central nervous system via three pathways: (1) in the vagus nerve and its branches; (2) within and alongside sympathetic efferent fiber pathways (sympathetic chain and splanchnic branches, including greater, lesser, least, thoracic, and lumbar branches); and (3) in the pelvic nerve (with parasympathetic efferents) and its branches. Passage through the peripheral ganglia occurs with potential synaptic contact (e.g., celiac, superior mesenteric, and hypogastric nerves). -

Neuromodulation Approaches for Cardiac Arrhythmias: Recent Advances
Current Cardiology Reports (2019) 21: 32 https://doi.org/10.1007/s11886-019-1120-1 INVASIVE ELECTROPHYSIOLOGY AND PACING (EK HEIST, SECTION EDITOR) Neuromodulation Approaches for Cardiac Arrhythmias: Recent Advances Veronica Dusi1,2 & Ching Zhu 2 & Olujimi A. Ajijola2 Published online: 18 March 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review This review aims to describe the latest advances in autonomic neuromodulation approaches to treating cardiac arrhythmias, with a focus on ventricular arrhythmias. Recent Findings The increasing understanding of neuronal remodeling in cardiac diseases has led to the development and improvement of novel neuromodulation therapies targeting multiple levels of the autonomic nervous system. Thoracic epidural anesthesia, spinal cord stimulation, stellate ganglion modulatory therapies, vagal stimulation, renal denervation, and interventions on the intracardiac nervous system have all been studied in preclinical models, with encouraging preliminary clinical data. Summary The autonomic nervous system regulates all the electrical processes of the heart and plays an important role in the pathophysiology of cardiac arrhythmias. Despite recent advances in the clinical application of cardiac neuromodulation, our comprehension of the anatomy and function of the cardiac autonomic nervous system is still limited. Hopefully in the near future, more preclinical data combined with larger clinical trials will lead to further improvements in neuromodulatory treatment for heart rhythm disorders. Keywords Cardiac autonomic nervous system . Ventricular arrhythmias . Neuromodulation . Stellate ganglion Introduction feedback loops that enable autonomic control of cardiac func- tion. The entire autonomic nervous system (ANS) aims to The heart is richly innervated by afferent as well as sympa- maintain cardiac homeostasis in the face of environmental thetic and parasympathetic efferent nerves. -

The Sympathetic and Parasympathetic Contributions to the Cardiac Plexus: a Fetal Study
Int. J. Morphol., 30(4):1569-1576, 2012. The Sympathetic and Parasympathetic Contributions to the Cardiac Plexus: a Fetal Study Contribuciones Simpáticas y Parasimpáticas al Plexo Cardíaco: Estudio Fetal B. Z. De Gama; L. Lazarus; P. Partab & K. S. Satyapal DE GAMA, B. Z.; LAZARUS, L.; PARTAB, P. & SATYAPAL, K. S. The sympathetic and parasympathetic contributions to the cardiac plexus: a fetal study. Int. J. Morphol., 30(4):1569-1576, 2012. SUMMARY: The cardiac plexus is formed by sympathetic nerves originating from the superior, middle, inferior cervical or cervicothoracic ganglia as well as from the first to the fifth thoracic ganglia. Furthermore, the vagus nerve and its counterpart, the recurrent laryngeal nerve supply the cardiac plexus with parasympathetic cardiac nerves. This investigation aimed to review and record the medial contributions of the cervical ganglia, first to fifth thoracic ganglia and medial contributions of the vagus and recurrent laryngeal nerves to the cardiac plexus. The study involved bilateral micro-dissection of forty cadaveric fetal specimens (n=80). The origins of sympathetic contributions to the cardiac plexus were described as either ganglionic, inter-ganglionic or from both the ganglion and the inter-ganglionic sympathetic chain. The number of cervical sympathetic ganglia varied from two to five in this study; the superior cervical ganglion was constant while the middle cervical, vertebral, inferior cervical or cervicothoracic ganglia were variable. The prevalence of cardiac nerves were as follows: superior cervical cardiac nerve (95%); middle cervical cardiac nerve (73%); vertebral cardiac nerve (41%); inferior cervical cardiac nerve (21%) and cervicothoracic cardiac nerve (24%). This investigation records the thoracic caudal limit of the thoracic sympathetic contributions to the cardiac plexus as the T5 ganglion. -

Review of the External Carotid Plexus: Anatomy, Function, and Clinical Manifestations
Review Article https://doi.org/10.5115/acb.20.308 pISSN 2093-3665 eISSN 2093-3673 Review of the external carotid plexus: anatomy, function, and clinical manifestations Shadi E. Razipour1, Sina Zarrintan2, Mansour Mathkour3, Joe Iwanaga3,4, Aaron S. Dumont3, R. Shane Tubbs1,3,4,5,6 1Department of Structural & Cellular Biology, Tulane University School of Medicine, New Orleans, LA, USA, 2Department of General & Vascular Surgery, Shahid Beheshti University of Medical Sciences, Tehran, Iran, 3Department of Neurosurgery, Tulane Center for Clinical Neurosciences, Tulane University School of Medicine, New Orleans, LA, 4Department of Neurology, Tulane Center for Clinical Neurosciences, Tulane University School of Medicine, New Orleans, LA, 5Department of Neurosurgery and Ochsner Neuroscience Institute, Ochsner Health System, New Orleans, LA, USA, 6Department of Anatomical Sciences, St. George’s University, St. George’s, Grenada, West Indies Abstract: The external carotid plexus is a combination of postganglionic sympathetic fibers derived from the superior cervical ganglion. This plexus travels along the external carotid artery and continues onto the artery’s branches. The external carotid plexus plays an important role in innervating the mid and lower face. Therefore, implications to the plexus may result in facial abnormalities. Herein, we review the anatomy, function, and review its clinical applications. Key words: External carotid plexus, Anatomy, General surgery, Sympathetic fiber, External carotid artery Received November 26, 2020; 1st Revised January 10, 2021; 2nd Revised February 19, 2021; Accepted February 25, 2021 Introduction returning superiorly to anastomose with the prior branch to the external carotid artery [2, 5] Branches of the plexus The external carotid plexus (Figs. 1 and 2) encapsulates partially continue onto the branches of the external carotid the external carotid artery and its branches to innervate the artery. -

Systematic and Comparative Morphologies of the Extrinsic
Zoologischer Anzeiger 252 (2013) 101–117 Contents lists available at SciVerse ScienceDirect Zoologischer Anzeiger journal homepage: www.elsevier.de/jcz Systematic and comparative morphologies of the extrinsic cardiac nervous system in lemurs (Primates: Strepsirrhini: Infraorder Lemuriformes, Gray, 1821) with evolutionary morphological implications Tomokazu Kawashima a,b,∗, Richard W. Thorington Jr. a, Fumi Sato b a Division of Mammals, Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, 10th & Constitution Ave., NW, Washington, DC 20013-7012, USA b Department of Anatomy, School of Medicine, Toho University, Omori-Nishi 5-21-16, Ota-ku, Tokyo 143-8540, Japan article info abstract Article history: The detailed systematic morphology of the extrinsic cardiac nervous system (ECNS) and its surrounding Received 26 July 2011 structures in lemurs, Lemuriformes, were examined using 18 sides of nine lemurs including all four Received in revised form 22 March 2012 families: Lemuridae, Indriidae, Lepilemuridae, and Cheirogaleidae. Although the general morphology of Accepted 4 April 2012 ECNS in lemurs is similar to that in Lorisiformes, several anatomical differences, such as the structural Available online 8 May 2012 variation of the cervicothoracic ganglion, the positional variation of the middle cervical ganglion, and Corresponding Editor: Carsten Lüter. the unusual appearance of the superior cardiac nerve originating from the superior cervical ganglion, demonstrate the diversity within Lemuriformes. In other words, the comparative anatomical findings of Keywords: Lemurs the wide variations in ECNS in the four families of Lemuriformes compared with the lower variations in Comparative anatomy the two families of Lorisiformes possibly reflect their evolutionary history and diversity, as is shown in Primate evolution the recent molecular phylogeny. -

Innervation of the Heart and Ductus Arteriosus and Other Features of the Autonomic Nervous System of the Full Term Human Fetus
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1954 Innervation of the Heart and Ductus Arteriosus and Other Features of the Autonomic Nervous System of the Full Term Human Fetus. Frank D. Allan Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Part of the Life Sciences Commons Recommended Citation Allan, Frank D., "Innervation of the Heart and Ductus Arteriosus and Other Features of the Autonomic Nervous System of the Full Term Human Fetus." (1954). LSU Historical Dissertations and Theses. 8086. https://digitalcommons.lsu.edu/gradschool_disstheses/8086 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. THE INNERVATION OF THE HEART AND DUCTUS ARTERIOSUS AND OTHER FEATURES OF THE AUTONOMIC NERVOUS SYSTEM OF THE FULL TERM HUMAN FETUS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agriculture and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Anatomy School of Medicine by Frank Duane Allan B.S., University of Utah, 1947 M.S., University of Utah, 1949 May, 1954 UMl Number: DP69464 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Characterization of the Intrinsic Cardiac Nervous System
Autonomic Neuroscience: Basic and Clinical 199 (2016) 3–16 Contents lists available at ScienceDirect Autonomic Neuroscience: Basic and Clinical journal homepage: www.elsevier.com/locate/autneu Review Characterization of the intrinsic cardiac nervous system Emily Wake, Kieran Brack Dr ⁎ Department of Cardiovascular Sciences, Cardiology Group, Glenfield Hospital, Groby Road, University of Leicester, LE3 9QP, United Kingdom Leicester NIHR Biomedical Research Unit in Cardiovascular Disease, Glenfield Hospital, Leicester LE3 9QP, United Kingdom article info abstract Article history: Heart disease is the number one cause of mortality in the developed world and it is well recognised that neural Received 4 May 2016 mechanisms are important in pathology. As well as peripheral autonomic nerves, there is a rich intrinsic inner- Received in revised form 29 June 2016 vation of the heart that includes cardiac ganglia, collectively termed ganglionic plexuses (GP). Understanding Accepted 3 August 2016 the role that the intrinsic cardiac nervous system (ICNS) play in controlling cardiac function and how it interacts with information between central command centers and its integration with sensory information from the myo- Keywords: Intrinsic cardiac nervous system cardium could prove crucial for prophylactic and corrective treatments of heart disease. This article in the timely Cardiac ganglia and important special issue on central and peripheral nervous control of the heart in Autonomic Neuroscience; Ganglionic plexuses Basic and Clinical will focus on -
The Discoverers of the Thoracic Cardiac Nerves
THE DISCOVERERS OF THE THORACIC CARDIAC NERVES By G. A. G. MITCHELL, Manchester University BECAUSE of their importance in connection with the surgical treatment of angina pectoris the thoracic cardiac nerves are small objects of great interest. It is therefore surprising that amongst the many articles and books dealing with the subject of cardiac innervation which have appeared since these nerves were first described, not one provides accurate information about their discovery. Judging from some of these writings, their very existence is not universally recognised, so it may be advisable to explain briefly that the cardiac plexus, amongst many other branches, receives slender contributions from the second, third, fourth and possibly fifth thoracic sympathetic ganglia and that these constitute the thoracic cardiac nerves. Apart from the works mentioned later, no details of the thoracic cardiac nerves have been found in any of the great anatomical mono- graphs, atlases and textbooks published during the nineteenth century. Early this century Mollard (1908) produced an elaborate monograph on the cardiac nerves, but he described none arising from the sympathetic trunks below the level of the first thoracic ganglia, although he quoted from the works of such men as E. H. Weber (1815) and G. Valentin (1843) who had found cardiac branches arising at a lower level. Those writing exhaustive monographs seldom succeed in assimilating com- pletely all the information contained in the works listed in their impressive bibliographies, and Mollard was no exception. J. Dogiel, either alone or in collaboration with others, published many papers on the heart over a period of about thirty years, but he too remained unaware of the existence of the thoracic cardiac nerves. -
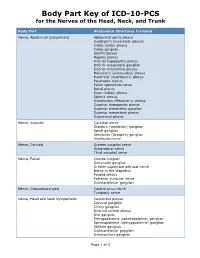
Body Part Key of ICD-10-PCS for the Nerves of the Head, Neck, and Trunk
Body Part Key of ICD-10-PCS for the Nerves of the Head, Neck, and Trunk Body Part Anatomical Structures Included Nerve, Abdominal Sympathetic Abdominal aortic plexus Auerbach’s (myenteric plexus) Celiac (solar) plexus Celiac ganglion Gastric plexus Hepatic plexus Inferior hypogastric plexus Inferior mesenteric ganglion Inferior mesenteric plexus Meissner’s (submucous) plexus Myenteric (Auerbach’s) plexus Pancreatic plexus Pelvic splanchnic nerve Renal plexus Solar (celiac) plexus Splenic plexus Submucous (Meissner’s) plexus Superior hypogastric plexus Superior mesenteric ganglion Superior mesenteric plexus Suprarenal plexus Nerve, Acoustic Cochlear nerve Scarpa’s (vestibular) ganglion Spiral ganglion Vestibular (Scarpa’s) ganglion Vestibular nerve Nerve, Cervical Greater occipital nerve Suboccipital nerve Third occipital nerve Nerve, Facial Chorda tympani Geniculate ganglion Greater superficial petrosal nerve Nerve to the stapedius Parotid plexus Posterior auricular nerve Submandibular ganglion Nerve, Glossopharyngeal Carotid sinus nerve Tympanic nerve Nerve, Head and Neck Sympathetic Cavernous plexus Cervical ganglion Ciliary ganglion Internal carotid plexus Otic ganglion Pterygopalatine (sphenopalatine) ganglion Sphenopalatine (pterygopalatine) ganglion Stellate ganglion Submandibular ganglion Submaxillary ganglion Page 1 of 3 Body Part Key of ICD-10-PCS for the Nerves of the Head, Neck, and Trunk Body Part Anatomical Structures Included Nerve, Lumbar Lumbosacral trunk Superior clunic (cluneal) nerve Nerve, Lumbar Sympathetic Lumbar