Characterization of the Intrinsic Cardiac Nervous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiac Sympathectomy and Spinal Cord Stimulation Attenuate Reflex-Mediated Norepinephrine Release During Ischemia Preventing Ventricular Fibrillation
Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation Jeffrey L. Ardell, … , J. Andrew Armour, Kalyanam Shivkumar JCI Insight. 2019. https://doi.org/10.1172/jci.insight.131648. Research In-Press Preview Cardiology Neuroscience Rationale: Reflex-mediated sympathoexcitation is central to the pathogenesis of arrhythmias and heart disease; neuraxial modulation can favorably attenuate these cardiac reflexes leading to cardioprotection. Objective: The purpose of this study was to define the mechanism by which cardiac neural decentralization and spinal cord stimulation (SCS) reduces ischemia-induced ventricular fibrillation (VF) and sudden cardiac death (SCD) by utilizing direct neurotransmitter measurements in the heart. Methods and Results: Direct measurement of norepinephrine (NE) levels in the left ventricular (LV) interstitial fluid (ISF) by microdialysis in response to transient left anterior descending coronary artery occlusion (CAO: 15 min) in anesthetized canines. Responses were studied with: (i) intact neuraxis and were compared to those in which the (ii) intrathoracic component of the cardiac neuraxis (stellate ganglia),(iii) the intrinsic cardiac neuronal (ICN) system were surgically delinked from the central nervous system versus (iv) subjects with intact neuraxis subjected to pre-emptive SCS (T1-T3 spinal level). With an intact neuraxis, animals with exaggerated NE-ISF levels in response to CAO were at increased risk for VF and SCD. During CAO there was a 152% increase in NE level when the entire neuraxis was intact compared to 114% following intrathoracic neuraxial decentralization (removal of the stellates) and 16% increase following ICN decentralization, when the entire heart and ICN was delinked from the other levels of the neuraxis. -

Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions
Hindawi International Journal of Rheumatology Volume 2020, Article ID 2919625, 13 pages https://doi.org/10.1155/2020/2919625 Review Article Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions Worku Abie Liyew Biomedical Science Department, School of Medicine, Debre Markos University, Debre Markos, Ethiopia Correspondence should be addressed to Worku Abie Liyew; [email protected] Received 25 April 2020; Revised 26 June 2020; Accepted 13 July 2020; Published 29 August 2020 Academic Editor: Bruce M. Rothschild Copyright © 2020 Worku Abie Liyew. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Lumbar disc degeneration is defined as the wear and tear of lumbar intervertebral disc, and it is mainly occurring at L3-L4 and L4-S1 vertebrae. Lumbar disc degeneration may lead to disc bulging, osteophytes, loss of disc space, and compression and irritation of the adjacent nerve root. Clinical presentations associated with lumbar disc degeneration and lumbosacral nerve lesion are discogenic pain, radical pain, muscular weakness, and cutaneous. Discogenic pain is usually felt in the lumbar region, or sometimes, it may feel in the buttocks, down to the upper thighs, and it is typically presented with sudden forced flexion and/or rotational moment. Radical pain, muscular weakness, and sensory defects associated with lumbosacral nerve lesions are distributed on -

'Voodoo' Death Revisited: the Modern Lessons of Neurocardiology†
KEYNOTE ADDRESS MARTIN A. SAMUELS, MD, DSc (hon), FAAN, MACP* Neurologist-in-Chief and Chairman Department of Neurology Brigham and Women’s Hospital Professor of Neurology Harvard Medical School Boston, MA ‘Voodoo’ death revisited: The modern lessons of neurocardiology† n 1942, Walter Bradford Cannon published a personal danger or threat of injury; (7) after danger is remarkable paper entitled “‘Voodoo’ Death,”1 in over; and (8) reunion, triumph, or happy ending. which he recounted anecdotal experiences, largely Common to all is that they involve events impossible I from the anthropology literature, of death from for the victim to ignore and to which the response is fright. These events, drawn from widely disparate parts overwhelming excitation, giving up, or both. of the world, had several features in common. They were In 1957, Carl Richter reported on a series of experi- all induced by an absolute belief that an external force, ments aimed at elucidating the mechanism of such as a wizard or medicine man, could, at will, cause Cannon’s “voodoo” death.3 Richter studied the length demise and that the victim himself had no power to alter of time domesticated rats could swim at various water this course. This perceived lack of control over a power- temperatures and found that at a water temperature of ful external force is the sine qua non for all the cases 93º these rats could swim for 60 to 80 minutes. recounted by Cannon, who postulated that death was However, if the animal’s whiskers were trimmed, it caused “by a lasting and intense action of the sym- would invariably drown within a few minutes. -

Neuromodulation Therapies for Intractable Angina: a Review
Research Article Clinics in Surgery Published: 06 Apr, 2021 Neuromodulation Therapies for Intractable Angina: A Review Patel S1* and Mammis A2 1Department of Neurosurgery, Rutgers New Jersey Medical School, USA 2Department of Neurosurgery, New York University School of Medicine, USA Abstract Background: Transcutaneous Electrical Nerve Stimulation (TENS) and Spinal Cord Stimulation (SCS) are neuromodulation therapies that have shown to be effective as treatments for the care of Intractable Angina. In addition, Stellate Ganglion Block (SGB), although not considered a neuromodulation therapy, has proven to be a successful interventional therapy. Objective: With the minimal research studying the use of neuromodulation therapies to treat intractable angina, this review looks to summarize the pre-existing data published and see which neuromodulation therapies have proven to be successful in treating patients with Intractable Angina. Methods: A literature search was done on PubMed, Google Scholars, and ResearchGate to discover 3 different neuromodulation therapies that have shown to be effective in the treatment of intractable angina. Factors that were searched for include safety, complications, and the application of the neuromodulation therapy. Results: 12 articles were analyzed with 3 three different neuromodulation therapies. For Spinal Cord Stimulation (SCS), 19 patients were implanted for SCS and the results found that both admission rate and hospital stay time were lower after SCS (0.97 vs. 0.27) and (8.3 days vs. 2.5 days). For Transcutaneous Electrical Nerve Stimulation (TENS), results have shown reduced frequency of anginal attacks, and increased work capacity. For Stellate Ganglion Block (SGB), the mean pain relief duration was 3.5 weeks. SBG has proven to be an alternative neuromodulation invasive strategy for the treatment of intractable angina. -
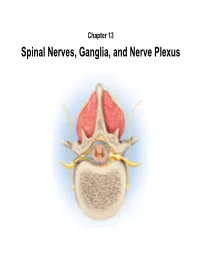
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Book of Abstracts
11rH CONGRESS OF THE INTERNATIONAL SOCIETY FOR AUTONOMIC NEUROSCIENCE BOOK OF JULY 25 - 27, 2019 I LOS ANGELES, CALIFORNIA ABSTRACTS Confocal microscopic image of a mouse dorsal root ganglion labeled with a multicolor adeno-associated virus-based approach. Neurons within the dorsal root ganglia are critical for processing sensory information from visceral organs. Image courtesy of Pradeep Rajendran and Rosemary Challis of the Shivkumar Lab at UCLA and the Gradinaru Lab at Caltech. isan2019.org 11rH CONGRESS OF THE INTERNATIONAL SOC IETY FOR AUTONOMIC NEUROSCIENCE JULY 25 - 27 , 2019 LOS ANGELES , CALIFORNIA TABLE OF CONTENTS ORAL PRESENTATIONS 4 POSTER PRESENTATIONS THURSDAY JULY 25 60 FRIDAY JULY 26 82 SATURDAY JULY 27 112 TECHNOLOGY SHOWCASE 142 AUTHOR INDEX 154 11TH CONGRESS OF THE INTERNATIONAL SOCIETY FOR AUTONOMIC NEUROSCIENCE JULY 25-27, 2019 | LOS ANGELES CALIFORNIA 2 ORAL PRESENTATIONS isan2019.org ORAL PRESENTATIONS SESSION: ENTERIC NEURAL CIRCUITRY IN MOUSE AND HUMAN COLON: THE CHANGING FACE OF MORPHOLOGY, FUNCTION AND THERAPEUTIC LEADS JULY 25, 2019 | 9:20 AM–11:20 AM ISAN19.003 ISAN19.005 REFINING ENTERIC NEURAL CIRCUITRY BY VISUALIZING THE HUMAN ENTERIC NERVOUS SYSTEM QUANTITATIVE MORPHOLOGY, TRANSCRIPTOMICS IN 3-DIMENSIONS: NEW WAYS TO THINK ABOUT AND FUNCTION IN MICE AUTONOMIC NEUROPATHY Marthe Howard, Andrea L. Kalinoski, Joseph F. Margiotta, Kahleb Graham1, Silvia Huerta Lopez1, Rajarshi Sengupta1, Samantha Mckee, Kalina Venkova, Alexander Hristov Archana Shenoy1, Christina M. Wright1, Sabine Schneider1, Michael D. Feldman2, Emma E. Furth3, Federico University of Toledo Health Sciences, Neurosciences, Toledo, 2 4 1 UNITED STATES OF AMERICA Valdivieso , Amanda Lemke , Benjamin J. Wilkins , Marthe Howard5, Pierre A. Russo6, Edward J. -

Association of Arrhythmia in Patients with Cervical Spondylosis: a Nationwide Population-Based Cohort Study
Journal of Clinical Medicine Article Association of Arrhythmia in Patients with Cervical Spondylosis: A Nationwide Population-Based Cohort Study Shih-Yi Lin 1,2, Wu-Huei Hsu 1,3, Cheng-Chieh Lin 1,4, Cheng-Li Lin 5,6, Chun-Hao Tsai 1,7, Chih-Hsueh Lin 1,4, Der-Cherng Chen 7, Tsung-Chih Lin 8, Chung-Y. Hsu 1 and Chia-Hung Kao 1,9,10,* ID 1 Graduate Institute of Biomedical Sciences and School of Medicine, College of Medicine, China Medical University, No. 2, Yuh-Der Road, Taichung 404, Taiwan; [email protected] (S.-Y.L.); [email protected] (W.-H.H.); [email protected] (C.-C.L.); [email protected] (C.-H.T.); [email protected] (C.-H.L.); [email protected] (C.-Y.H.) 2 Division of Nephrology and Kidney Institute, China Medical University Hospital, Taichung 404, Taiwan 3 Division of Pulmonary and Critical Care Medicine, China Medical University Hospital and China Medical University, Taichung 404, Taiwan 4 Department of Family Medicine, China Medical University Hospital, Taichung 404, Taiwan 5 Management Office for Health Data, China Medical University Hospital, Taichung 404, Taiwan; [email protected] 6 College of Medicine, China Medical University, Taichung 404, Taiwan 7 Department of Orthopedics, China Medical University Hospital, Taichung 404, Taiwan; [email protected] 8 Department of Orthopedics, St. Martin De Porres Hospital, Chiayi 600, Taiwan; [email protected] 9 Department of Nuclear Medicine, China Medical University Hospital, Taichung 404, Taiwan 10 Department of Bioinformatics and Medical Engineering, Asia University, Taichung 413, Taiwan * Correspondence: [email protected]; Tel.: +886-4-2205-2121 (ext. -

A Step Towards Stereotactic Navigation During Pelvic Surgery: 3D Nerve Topography
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository Surgical Endoscopy and Other Interventional Techniques https://doi.org/10.1007/s00464-018-6086-3 A step towards stereotactic navigation during pelvic surgery: 3D nerve topography A. R. Wijsmuller1,2 · C. Giraudeau3 · J. Leroy4 · G. J. Kleinrensink5 · E. Rociu6 · L. G. Romagnolo7 · A. G. F. Melani7,8,9 · V. Agnus2 · M. Diana3 · L. Soler3 · B. Dallemagne2 · J. Marescaux2 · D. Mutter2 Received: 10 May 2017 / Accepted: 1 February 2018 © The Author(s) 2018. This article is an open access publication Abstract Background Long-term morbidity after multimodal treatment for rectal cancer is suggested to be mainly made up by nerve- injury-related dysfunctions. Stereotactic navigation for rectal surgery was shown to be feasible and will be facilitated by highlighting structures at risk of iatrogenic damage. The aim of this study was to investigate the ability to make a 3D map of the pelvic nerves with magnetic resonance imaging (MRI). Methods A systematic review was performed to identify a main positional reference for each pelvic nerve and plexus. The nerves were manually delineated in 20 volunteers who were scanned with a 3-T MRI. The nerve identifiability rate and the likelihood of nerve identification correctness were determined. Results The analysis included 61 studies on pelvic nerve anatomy. A main positional reference was defined for each nerve. On MRI, the sacral nerves, the lumbosacral plexus, and the obturator nerve could be identified bilaterally in all volunteers. The sympathetic trunk could be identified in 19 of 20 volunteers bilaterally (95%). -

Human Consciousness: the Universal Heart Based Resonant Frequencies and the Massive Ecosystems Hierarchy
ISSN: 2641-1911 DOI: 10.33552/ANN.2020.09.000709 Archives in Neurology & Neuroscience Mini Review Copyright © All rights are reserved by Abdullah A Alabdulgader Human Consciousness: The Universal Heart Based Resonant Frequencies and the Massive ecosystems Hierarchy Abdullah A Alabdulgader* Congenital Cardiologist & Invasive Electro physiologist, Prince Sultan Cardiac Center, Saudi Arabia *Corresponding author: Abdullah Al Abdulgader, Congenital Cardiologist & Received Date: October 15, 2020 Invasive Electro physiologist, Prince Sultan Cardiac Center, P.O.Box 9596, Hofuf, Al-Ahsa 31982, Saudi Arabia. Published Date: November 02, 2020 Mini Review the 21st century, are still not investigated in an integral had became evident, the dawn of which emerged in respectful Few scientific dilemmas in the beginning of the third decade of publications from KOO and scientific collaborates research. There is compelling scientific and rational evidence to convince scientific comprehensive approach compatible with the era scientific communities that the nature of consciousness involves dynamics advances. Human Consciousness, its origin, nature and the delicate inside the skull but essentially much beyond it in extreme symphony orchestrating its execution remains at the top of the list adopted is based on holistic perspective and understanding of dimensions between the skull and the sky. The approach we of the elusive sciences of the human history. Intuitively, our ancestors refer to the human heart as the seat of soul and the center human consciousness -

35. Lumbar Plexus. Sacral Plexus. Coccygeal Plexus
GUIDELINES Students’ independent work during preparation to practical lesson Academic discipline HUMAN ANATOMY Topic LUMBAR PLEXUS. SACRAL PLEXUS. COCCYGEAL PLEXUS 1. Relevance of the topic Lumbar, sacral and coccygeal plexuses innervate the skin of the abdomen, lower back and lower extremities and all the muscles of the lower limbs. Acquired knowledge is the basis for many fields of practical medicine, such as neurology, surgery and traumatology. 2. Specific objectives After the lesson the student should know and be able to: - describe the sources of the formation of the lumbar plexus; - classify the nerves of the lumbar plexus; - to be able to demonstrate and define the branches of the lumbar plexus; - describe sources of sacral plexus formation; - classify sacral plexus nerves; - be able to demonstrate and identify short and long branches of the sacral plexus; - describe the sources of formation coccygeal plexus; - classify coccygeal plexus nerves; - be able to demonstrate and identify branches of coccygeal plexus; - to explain the innervation of muscles and skin in the areas of the lower back and lower extremity. 3. Basic level of preparation For practical this lesson a student should know and be able: - to know the anatomy of the spine, pelvis, lower extremities; - to analyze and show large and small pelvis, their bones; - to analyze and demonstrate bones and joints of the lower limbs; - to demonstrate muscles of the abdomen, perineum, pelvic girdle and lower limbs; - to know the anatomy (external and internal structure) of the spinal cord; - to know the spinal nerve anatomy. 4. Tasks for independent work during preparation for the classes 4.1. -

Management of Metastatic Tumors Invading the Peripheral Nervous System
Neurosurg Focus 22 (6):E14, 2007 Management of metastatic tumors invading the peripheral nervous system JOHN GACHIANI, M.D.,1 DANIEL H. KIM, M.D.,3 ADRIANE NELSON, M.D.,2 AND DAVID KLINE, M.D.1 Departments of 1Neurosurgery and 2Pathology, Louisiana State University Health Sciences Center; 3Department of Neurosurgery, Ochsner Clinic Foundation, New Orleans, Louisiana Object. The authors present the results of a retrospective review of 37 surgically treated metastases to nerve (malignant peripheral non–neural sheath nerve tumors). Tumor frequencies, presentations, management, and prognosis are discussed. Methods. Thirty-seven patients who were treated for metastases to nerve between 1969 and 2006 at the Louisiana State University Health Sciences Center were identified in a review of patient records. Notes regarding patient history and physical examination findings were reviewed to provide informa- tion on presenting symptoms and signs. Imaging and histopathological examination results were also reviewed. Cases were analyzed depending on the primary tumor and the location of metastasis. Results. There included 37 surgically treated lesions, 16 of which originated in the breast and 10 of which originated in the lung. In two cases melanomas had metastasized to nerve, and one tumor each had metastasized from the bladder, rectum, skin, head and neck, and thyroid, and from a primary Ewing sarcoma. There was a single lymphoma that had metastasized to the radial nerve and one chor- doma and one osteosarcoma, each of which had metastasized to the brachial plexus. Conclusions. The nervous system is involved in numerous ways by oncological process. Direct involvement of the peripheral nervous system occurs mostly from direct extension, although it occa- sionally occurs because of distant spread from the primary tumor to nerve. -

Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review
Journal of Clinical Medicine Review Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review Jakub S. G ˛asior 1,* , Antonio Roberto Zamunér 2 , Luiz Eduardo Virgilio Silva 3 , Craig A. Williams 4 , Rafał Baranowski 5, Jerzy Sacha 6,7 , Paulina Machura 8, Wacław Kochman 9 and Bo˙zenaWerner 10 1 Faculty of Medical Sciences and Health Sciences, Kazimierz Pulaski University of Technology and Humanities, 26-600 Radom, Poland 2 Departamento de Kinesiología, Universidad Católica del Maule, 3480112 Talca, Maule, Chile; [email protected] 3 Department of Internal Medicine, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo 14049-900, Brazil; [email protected] 4 Children’s Health and Exercise Research Centre, Sport and Health Sciences, College of Life and Environmental Sciences, University of Exeter, St Luke’s Campus, Exeter EX1 2LU, UK; [email protected] 5 Department of Heart Rhythm Disorders, National Institute of Cardiology, 04-628 Warsaw, Poland; [email protected] 6 Faculty of Physical Education and Physiotherapy, Opole University of Technology, 45-758 Opole, Poland; [email protected] 7 Department of Cardiology, University Hospital in Opole, University of Opole, 45-401 Opole, Poland 8 Department of Gynaecological Endocrinology, Medical University of Warsaw, 00-950 Warsaw, Poland; [email protected] 9 Clinical Department of Cardiology at Bielanski Hospital, National Institute of Cardiology, 01-809 Warsaw, Poland; [email protected] 10 Department of Pediatric Cardiology and General Pediatrics, Medical University of Warsaw, 02-091 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-793-199-222 Received: 18 March 2020; Accepted: 13 April 2020; Published: 16 April 2020 Abstract: Cardiac autonomic dysfunction has been reported in patients with cerebral palsy (CP).