HISTOLOGY & CELL BIOLOGY Mr. BABATUNDE, D.E DIGESTIVE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pancreatic B-Cell Replacement: Advances in Protocols Used for Differentiation of Pancreatic Progenitors to B-Like Cells
FOLIA HISTOCHEMICA REVIEW ET CYTOBIOLOGICA Vol. 57, No. 3, 2019 pp. 101–115 Pancreatic b-cell replacement: advances in protocols used for differentiation of pancreatic progenitors to b-like cells Muhammad Waseem Ghani1, Li Ye1, Zhao Yi1, Hammad Ghani2, Muhammad Waseem Birmani1, Aamir Nawab1, Lang Guan Cun1, Liu Bin1, Xiao Mei1 1Department of Livestock Production and Management, Agricultural College, Guangdong Ocean University, Zhanjiang, Guangdong, China 2Nawaz Sharif Medical College University of Gujrat, Punjab, Pakistan Abstract Insulin-producing cells derived from in vitro differentiation of stem cells and non-stem cells by using different factors can spare the need for genetic manipulation and provide a cure for diabetes. In this context, pancreatic progenitors differentiating to b-like cells garner increasing attention as b-cell replacement source. This kind of cell therapy has the potential to cure diabetes, but is still on its way of being clinically useful. The primary restriction for in vitro production of mature and functional b-cells is developing a physiologically relevant in vitro culture system which can mimic in vivo pathways of islet development. In order to achieve this target, different approaches have been attempted for the differentiation of pancreatic stem/progenitor cells to b-like cells. Here, we will review some of the state-of-the-art protocols for the differentiation of pancreatic progenitors and differentiated pancreatic cells into b-like cells with a focus on pancreatic duct cells. (Folia Histochemica et Cytobiologica -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr
Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr. Heather Yule ([email protected]) October 21, 2014 Slides for study: 75 (Salivary Gland), 355 (Pancreas Tail), 48 (Atrophic Mammary Gland), 49 (Active Mammary Gland) and 50 (Resting Mammary Gland) Electron micrographs for : study EM: Serous acinus in parotid gland EM: Mucous acinus in mixed salivary gland EM: Pancreatic acinar cell Main Objective: Understand key histological features of glandular epithelium and relate structure to function. Specific Objectives: 1. Describe key histological differences between endocrine and exocrine glands including their development. 2. Compare three modes of secretion in glands; holocrine, apocrine and merocrine. 3. Explain the functional significance of polarization of glandular epithelial cells. 4. Define the terms parenchyma, stroma, mucous acinus, serous acinus and serous a demilune and be able to them identify in glandular tissue. 5. Distinguish exocrine and endocrine pancreas. 6. Compare the histology of resting, lactating and postmenopausal mammary glands. Keywords: endocrine gland, exocrine gland, holocrine, apocrine, merocrine, polarity, parenchyma, stroma, acinus, myoepithelial cell, mucous gland, serous gland, mixed or seromucous gland, serous demilune, exocrine pancreas, endocrine pancreas (pancreatic islets), resting mammary gland, lactating mammary gland, postmenopausal mammary gland “This copy is made solely for your personal use for research, private study, education, parody, satire, criticism, or review -

Oral Cavity Histology Histology > Digestive System > Digestive System
Oral Cavity Histology Histology > Digestive System > Digestive System Oral Cavity LINGUAL PAPILLAE OF THE TONGUE Lingual papillae cover 2/3rds of its anterior surface; lingual tonsils cover its posterior surface. There are three types of lingual papillae: - Filiform, fungiform, and circumvallate; a 4th type, called foliate papillae, are rudimentary in humans. - Surface comprises stratified squamous epithelia - Core comprises lamina propria (connective tissue and vasculature) - Skeletal muscle lies deep to submucosa; skeletal muscle fibers run in multiple directions, allowing the tongue to move freely. - Taste buds lie within furrows or clefts between papillae; each taste bud comprises precursor, immature, and mature taste receptor cells and opens to the furrow via a taste pore. Distinguishing Features: Filiform papillae • Most numerous papillae • Their role is to provide a rough surface that aids in chewing via their keratinized, stratified squamous epithelia, which forms characteristic spikes. • They do not have taste buds. Fungiform papillae • "Fungi" refers to its rounded, mushroom-like surface, which is covered by stratified squamous epithelium. Circumvallate papillae • Are also rounded, but much larger and more bulbous. • On either side of the circumvallate papillae are wide clefts, aka, furrows or trenches; though not visible in our sample, serous Ebner's glands open into these spaces. DENTITION Comprise layers of calcified tissues surrounding a cavity that houses neurovascular structures. Key Features Regions 1 / 3 • The crown, which lies above the gums • The neck, the constricted area • The root, which lies within the alveoli (aka, sockets) of the jaw bones. • Pulp cavity lies in the center of the tooth, and extends into the root as the root canal. -
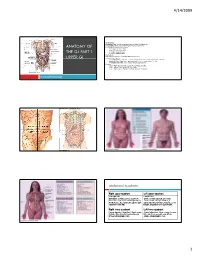
Anatomy of the G.I Part 1 Upper Gi
4/14/2009 Four Quadrants: •Midsagittal Plane: Vertical line going through the middle of the abdomen. •Transumbilical Plane: Horizontal line going through the umbilicus. ANATOMY OF •Four Quadrants based on those planes: •Right Upper Quadrant: RUQ •Right Lower Quadrant: RLQ •Left Upper Quadrant: LUQ THE G.I PART 1 •Left Lower Quadrant: LLQ Nine Regions: •Vertical lines of division: Left and Right Mid-Clavicular Lines UPPER GI •Horizontal lines of division: •Transpyloric Plane: Sometimes used. It is halfway between the jugular notch and the pubic bone. •Subcostal Plane: Upper plane, passing through the inferior-most margin of the ribs. •Transtubercular Plane: The line transversing the pubic tubercle. •Divisions: •Upper: Right Hypochondriac, Epigastric, Left Hypochondriac •Middle: Right Lumbar, Umbilical, Left Lumbar •Lower: Right Inguinal, Hypogastric (Suprapubic), Left Inguinal D.HAMMOUDI.MD Abdominal quadrants Right upper quadrant Left upper quadrant Liver right lobe Liver left lobe Gallbladder, stomach, pylorus, doudenum, Spleen, stomach, jejunum, prox ileum, Pancreas head, R suprarenal gland , R kidney , pancreas body and tail , left kidney, L R colic flexure, Ascending colon superior part, suprarenal, left colic flexure, Transverse colon Transvrse colon R half. left part, descending colon superior part. Right lower quadrant Left lower quadrant Cecum, Appendix, Ileum, Asc. Colon, R ovary, Sigmoid colon, Desc. Colon, L ovary, L uterine R uterine tube, R ureter, R spermatic cord, tube, L ureter, L spermatic cord, Uterus Uterus, Urinary bladder (full) enlarge, Urinary bladder ( full). 1 4/14/2009 Anatomy of the Mouth and Throat 8 Mouth: lips non-keratinized therefore Oral Cavity (mouth) evaporation occurs, must lick lips Entrance to the GI tract. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Interaction of Stellate Cells with Pancreatic Carcinoma Cells
Cancers 2010, 2, 1661-1682; doi:10.3390/cancers2031661 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Interaction of Stellate Cells with Pancreatic Carcinoma Cells Hansjörg Habisch 1, Shaoxia Zhou 1, Marco Siech 2 and Max G. Bachem 1,* 1 Department Clinical Chemistry and Central Laboratory, University of Ulm, Germany; E-Mails: [email protected] (H.H); [email protected] (S.Z.) 2 Department of surgery, Ostalbklinikum Aalen, Germany; E-Mail: [email protected] (M.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-731-500-67500/01; Fax: +49-731-500-67506. Received: 28 July 2010; in revised form: 2 September 2010 / Accepted: 2 September 2010 / Published: 9 September 2010 Abstract: Pancreatic cancer is characterized by its late detection, aggressive growth, intense infiltration into adjacent tissue, early metastasis, resistance to chemo- and radiotherapy and a strong ―desmoplastic reaction‖. The dense stroma surrounding carcinoma cells is composed of fibroblasts, activated stellate cells (myofibroblast-like cells), various inflammatory cells, proliferating vascular structures, collagens and fibronectin. In particular the cellular components of the stroma produce the tumor microenvironment, which plays a critical role in tumor growth, invasion, spreading, metastasis, angiogenesis, inhibition of anoikis, and chemoresistance. Fibroblasts, myofibroblasts and activated stellate cells produce the extracellular matrix components and are thought to interact actively with tumor cells, thereby promoting cancer progression. In this review, we discuss our current understanding of the role of pancreatic stellate cells (PSC) in the desmoplastic response of pancreas cancer and the effects of PSC on tumor progression, metastasis and drug resistance. -

Normal Pancreatic Function 1. What Are the Functions of the Pancreas?
Normal Pancreatic Function Stephen J. Pandol Cedars-Sinai Medical Center and Department of Veterans Affairs Los Angeles, California USA [email protected] Version 1.0, June 13, 2015 [DOI: 10.3998/panc.2015.17] 1. What are the functions of the This chapter presents processes underlying the functions of the exocrine pancreas with pancreas? references to how specific abnormalities of the The pancreas has both exocrine and endocrine pancreas can lead to disease states. function. This chapter is devoted to the exocrine functions of the pancreas. The exocrine function 2. Where is the pancreas located? is devoted to secretion of digestive enzymes, ions and water into the intestine of the gastrointestinal The illustration in Figure 1 demonstrates the (GI) tract. The digestive enzymes are necessary anatomical relationships between the pancreas for converting a meal into molecules that can be and organs surrounding it in the abdomen. The absorbed across the surface lining of the GI tract regions of the pancreas are the head, body, tail into the body. Of note, there are digestive and uncinate process (Figure 2). The distal end enzymes secreted by our salivary glands, of the common bile duct passes through the head stomach and surface epithelium of the GI tract of the pancreas and joins the pancreatic duct as it that also contribute to digestion of a meal. enters the intestine (Figure 2). Because the bile However, the exocrine pancreas is necessary for duct passes through the pancreas before entering most of the digestion of a meal and without it the intestine, diseases of the pancreas such as a there is a substantial loss of digestion that results cancer at the head of the pancreas or swelling in malnutrition. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON REGULATION OF THE SYNTHESIS OF TISSUE INHIBITORS OF METALLOPROTEINASE-1 AND –2 BY HEPATIC AND PANCREATIC STELLATE CELLS Dr Peter Raymond McCrudden B.Sc.(Hons) MBBS FRCP Submitted for the degree of MD Faculty of Medicine, Health and Biological Sciences June 2010 For Ute, Nicolas, Katia and India 2 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF MEDICINE, HEALTH AND LIFE SCIENCES (MHLS) SCHOOL OF MEDICINE DIVISION OF INFECTION, INFLAMMATION AND IMMUNITY Doctor of Medicine REGULATION OF THE SYNTHESIS OF TISSUE INHIBITORS OF METALLOPROTEINASE-1 AND –2 BY HEPATIC AND PANCREATIC STELLATE By Dr Peter Raymond McCrudden Hepatic stellate cells (HSC) play a central role in fibrosis development by production of extracellular matrix and also by secretion of matrix metalloproteinases (MMPs) and Tissue inhibitors of metalloproteinases (TIMPs) including TIMP-2 and MMP-2. -

Glandular-Epithelium.Pdf
Glandular Epithelium Glands • Definition: • “Glandular epithelia are tissues formed by cells specialized to produce secretion.” or • “An aggregation of gland cells into a definite structure for the purpose of secretion. Glandular epithelial cells may synthesize, store, and secrete: • Proteins (e.g; pancreas), • Lipids (e.g; adrenal, sebaceous glands), • Complexes of carbohydrates and proteins (e.g; salivary glands). • The mammary glands secrete all 3 substances. Development of glands • Formation of glands from covering epithelia. • Epithelial cells proliferate and penetrate connective tissue followed by further differerntiation. • They may–or may not–maintain contact with the surface. • When contact is maintained, exocrine glands are formed; • Without contact, endocrine glands are formed. Classification of glands • Glands are generally classified into two major groups: • Exocrine glands (Gr. Exo, outside,+ krinein, to separate). • Release their products onto an epithelial surface, either directly or through a duct e.g; the salivary glands, sweat glands, mammary glands. • Endocrine glands (Gr, endon, within,+ krinein). • Release their products ( hormones) into the blood stream, e.g; thyroid gland, parathyroid glands, pituitary gland, Adrenal glands. • Mixed variety: Some glands possess both exocrine and endocrine function. e.g; pancreas, liver cells. Exocrine glands secrete substances to specific organ via duct systems. Mammary glands Sweat glands • The cells of endocrine glands can be arranged in cords or in follicles. • The lumens of the follicles accumulate large quantities of secretions . Mixed gland: Pancreas Contains both endocrine and exocrine parts. Mixed gland: Pancreas Mixed gland: Pancreas Mixed gland: Liver Mixed gland: Liver General histological structure of gland General histological structure of gland • Externally a gland is surrounded by a dense layer of connective tissue which forms capsule of the gland. -

Extensive Pancreas Regeneration Following Acinar-Specific
GASTROENTEROLOGY 2011;141:1463–1472 Extensive Pancreas Regeneration Following Acinar-Specific Disruption of Xbp1 in Mice DAVID A. HESS,* SEAN E. HUMPHREY,* JEFF ISHIBASHI,* BARBARA DAMSZ,* ANN–HWEE LEE,‡ LAURIE H. GLIMCHER,‡ and STEPHEN F. KONIECZNY* *Department of Biological Sciences and the Purdue Center for Cancer Research, Purdue University, West Lafayette, Indiana; and ‡Department of Immunology and Infectious Diseases, Harvard School of Public Health, and Department of Medicine, Harvard Medical School, Boston, Massachusetts and survival of acinar cells is the management of their See editorial on page 1155. extensive protein synthesis/processing machinery, which uses regulatory pathways involving the Golgi, intermediate BACKGROUND & AIMS: Progression of diseases of the transport vesicles, plasma membrane components, and the exocrine pancreas, which include pancreatitis and cancer, is endoplasmic reticulum (ER). Among these organelles, the associated with increased levels of cell stress. Pancreatic aci- ER ensures that newly synthesized secretory and transmem- nar cells are involved in development of these diseases and, brane proteins are properly folded and modified before ex- because of their high level of protein output, they require an portation. In cells with high protein synthesis demands,2,3 efficient, unfolded protein response (UPR) that mediates with specific differentiation requirements,2,4,5 or that are recovery from endoplasmic reticulum (ER) stress following subject to environmental or physiologic alterations, the abil- the accumulation of misfolded proteins. METHODS: To ity of the protein-folding machinery to handle the synthetic study recovery from ER stress in the exocrine organ, we load can reach an imbalance, setting in motion a series of generated mice with conditional disruption of Xbp1 (a prin- intracellular signaling pathways collectively known as the cipal component of the UPR) in most adult pancreatic aci- unfolded protein response (UPR).6–8 Activation of the UPR nar cells (Xbp1fl/fl).