Study the Effect of Age on Histochemical, Histometrical and Immune Histological Finding of Submandibular Gland in Buffalo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr
Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr. Heather Yule ([email protected]) October 21, 2014 Slides for study: 75 (Salivary Gland), 355 (Pancreas Tail), 48 (Atrophic Mammary Gland), 49 (Active Mammary Gland) and 50 (Resting Mammary Gland) Electron micrographs for : study EM: Serous acinus in parotid gland EM: Mucous acinus in mixed salivary gland EM: Pancreatic acinar cell Main Objective: Understand key histological features of glandular epithelium and relate structure to function. Specific Objectives: 1. Describe key histological differences between endocrine and exocrine glands including their development. 2. Compare three modes of secretion in glands; holocrine, apocrine and merocrine. 3. Explain the functional significance of polarization of glandular epithelial cells. 4. Define the terms parenchyma, stroma, mucous acinus, serous acinus and serous a demilune and be able to them identify in glandular tissue. 5. Distinguish exocrine and endocrine pancreas. 6. Compare the histology of resting, lactating and postmenopausal mammary glands. Keywords: endocrine gland, exocrine gland, holocrine, apocrine, merocrine, polarity, parenchyma, stroma, acinus, myoepithelial cell, mucous gland, serous gland, mixed or seromucous gland, serous demilune, exocrine pancreas, endocrine pancreas (pancreatic islets), resting mammary gland, lactating mammary gland, postmenopausal mammary gland “This copy is made solely for your personal use for research, private study, education, parody, satire, criticism, or review -

Oral Cavity Histology Histology > Digestive System > Digestive System
Oral Cavity Histology Histology > Digestive System > Digestive System Oral Cavity LINGUAL PAPILLAE OF THE TONGUE Lingual papillae cover 2/3rds of its anterior surface; lingual tonsils cover its posterior surface. There are three types of lingual papillae: - Filiform, fungiform, and circumvallate; a 4th type, called foliate papillae, are rudimentary in humans. - Surface comprises stratified squamous epithelia - Core comprises lamina propria (connective tissue and vasculature) - Skeletal muscle lies deep to submucosa; skeletal muscle fibers run in multiple directions, allowing the tongue to move freely. - Taste buds lie within furrows or clefts between papillae; each taste bud comprises precursor, immature, and mature taste receptor cells and opens to the furrow via a taste pore. Distinguishing Features: Filiform papillae • Most numerous papillae • Their role is to provide a rough surface that aids in chewing via their keratinized, stratified squamous epithelia, which forms characteristic spikes. • They do not have taste buds. Fungiform papillae • "Fungi" refers to its rounded, mushroom-like surface, which is covered by stratified squamous epithelium. Circumvallate papillae • Are also rounded, but much larger and more bulbous. • On either side of the circumvallate papillae are wide clefts, aka, furrows or trenches; though not visible in our sample, serous Ebner's glands open into these spaces. DENTITION Comprise layers of calcified tissues surrounding a cavity that houses neurovascular structures. Key Features Regions 1 / 3 • The crown, which lies above the gums • The neck, the constricted area • The root, which lies within the alveoli (aka, sockets) of the jaw bones. • Pulp cavity lies in the center of the tooth, and extends into the root as the root canal. -
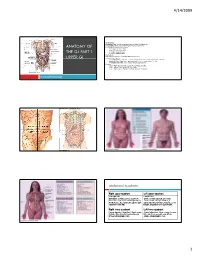
Anatomy of the G.I Part 1 Upper Gi
4/14/2009 Four Quadrants: •Midsagittal Plane: Vertical line going through the middle of the abdomen. •Transumbilical Plane: Horizontal line going through the umbilicus. ANATOMY OF •Four Quadrants based on those planes: •Right Upper Quadrant: RUQ •Right Lower Quadrant: RLQ •Left Upper Quadrant: LUQ THE G.I PART 1 •Left Lower Quadrant: LLQ Nine Regions: •Vertical lines of division: Left and Right Mid-Clavicular Lines UPPER GI •Horizontal lines of division: •Transpyloric Plane: Sometimes used. It is halfway between the jugular notch and the pubic bone. •Subcostal Plane: Upper plane, passing through the inferior-most margin of the ribs. •Transtubercular Plane: The line transversing the pubic tubercle. •Divisions: •Upper: Right Hypochondriac, Epigastric, Left Hypochondriac •Middle: Right Lumbar, Umbilical, Left Lumbar •Lower: Right Inguinal, Hypogastric (Suprapubic), Left Inguinal D.HAMMOUDI.MD Abdominal quadrants Right upper quadrant Left upper quadrant Liver right lobe Liver left lobe Gallbladder, stomach, pylorus, doudenum, Spleen, stomach, jejunum, prox ileum, Pancreas head, R suprarenal gland , R kidney , pancreas body and tail , left kidney, L R colic flexure, Ascending colon superior part, suprarenal, left colic flexure, Transverse colon Transvrse colon R half. left part, descending colon superior part. Right lower quadrant Left lower quadrant Cecum, Appendix, Ileum, Asc. Colon, R ovary, Sigmoid colon, Desc. Colon, L ovary, L uterine R uterine tube, R ureter, R spermatic cord, tube, L ureter, L spermatic cord, Uterus Uterus, Urinary bladder (full) enlarge, Urinary bladder ( full). 1 4/14/2009 Anatomy of the Mouth and Throat 8 Mouth: lips non-keratinized therefore Oral Cavity (mouth) evaporation occurs, must lick lips Entrance to the GI tract. -

Glandular-Epithelium.Pdf
Glandular Epithelium Glands • Definition: • “Glandular epithelia are tissues formed by cells specialized to produce secretion.” or • “An aggregation of gland cells into a definite structure for the purpose of secretion. Glandular epithelial cells may synthesize, store, and secrete: • Proteins (e.g; pancreas), • Lipids (e.g; adrenal, sebaceous glands), • Complexes of carbohydrates and proteins (e.g; salivary glands). • The mammary glands secrete all 3 substances. Development of glands • Formation of glands from covering epithelia. • Epithelial cells proliferate and penetrate connective tissue followed by further differerntiation. • They may–or may not–maintain contact with the surface. • When contact is maintained, exocrine glands are formed; • Without contact, endocrine glands are formed. Classification of glands • Glands are generally classified into two major groups: • Exocrine glands (Gr. Exo, outside,+ krinein, to separate). • Release their products onto an epithelial surface, either directly or through a duct e.g; the salivary glands, sweat glands, mammary glands. • Endocrine glands (Gr, endon, within,+ krinein). • Release their products ( hormones) into the blood stream, e.g; thyroid gland, parathyroid glands, pituitary gland, Adrenal glands. • Mixed variety: Some glands possess both exocrine and endocrine function. e.g; pancreas, liver cells. Exocrine glands secrete substances to specific organ via duct systems. Mammary glands Sweat glands • The cells of endocrine glands can be arranged in cords or in follicles. • The lumens of the follicles accumulate large quantities of secretions . Mixed gland: Pancreas Contains both endocrine and exocrine parts. Mixed gland: Pancreas Mixed gland: Pancreas Mixed gland: Liver Mixed gland: Liver General histological structure of gland General histological structure of gland • Externally a gland is surrounded by a dense layer of connective tissue which forms capsule of the gland. -

HISTOLOGY & CELL BIOLOGY Mr. BABATUNDE, D.E DIGESTIVE
HISTOLOGY & CELL BIOLOGY Mr. BABATUNDE, D.E DIGESTIVE GLANDS I EXTRINSIC GLANDS ❖ Of the digestive system are located outside the wall of the alimentary canal and deliver their secretion into the lumen via a system of ducts. ❖ Provide enzymes, buffers, emulsifiers, and lubricants for the digestive tract, as well as hormones, proteins, globulins, and numerous additional products for the remainder of the body. ❖ Are the salivary glands (parotid, sublingual, and submandibular), the pancreas, and the liver. MAJOR SALIVARY GLANDS Parotid Gland Classification ❖ Purely serous, compound, tubuloalveolar gland. Capsule ❖ Formed by the continuation of the superficial cervical fascia, is mostly collagenous in nature. ❖ Forms broad bands of trabeculae (septa) that subdivide the gland into lobes and lobules. Trabeculae ❖ Convey blood and lymph vessels, ducts, and nerves into the substance of the gland. ❖ Often contain fat cells in older individuals. Acini ❖ Although are said to be composed of purely serous cells, they are actually seromucous in character. ❖ Are surrounded by myoepithelial cells. ❖ Their center contains the lumen. Parotid gland : only contains serous secretory units Figure 16—2. Photomicrograph of a parotid gland. Its secretory portion consists of serous amylase—producing cells that store this enzyme in secretory granules. Intralobular (intercalated and striated) ducts are also present. Pararosaniline— toluidine blue (PT) stain. Medium magnification. Acinar Cells ❖ Pyramidal cells, whose apical aspects usually contain secretory granules. ❖ Nucleus is round and basally located. ❖ Cytoplasm contains extensive rough endoplasmic reticulum, a well-developed Golgi apparatus, and numerous mitochondria. Myoepithelial Cells ❖ Are stellate shaped. ❖ Their cytoplasm is difficult to discern with the light microscope. ❖ Electron micrographs demonstrate that they resemble smooth muscle cells and are contractile. -

This Lab Has an Associated Homework Assignment That Is Posted on Blackboard. If You Have Not Done This Assignment Already, It W
LABORATORY 4 - EPITHELIUM - (first of two laboratory sessions) This lab has an associated homework assignment that is posted on Blackboard. If you have not done this assignment already, it would make this lab more efficient if you did so. OBJECTIVES: LIGHT MICROSCOPY - Recognize: Simple epithelia including: simple squamous, cuboidal, columnar and pseudostratified columnar epithelium Stratified epithelia including: stratified squamous (keratinized and non-keratinized), stratified cuboidal, stratified columnar and transitional epithelium Surface modifications including: apical surface, striated border, cilia, stereocilia, "intercellular" bridges, basal surface, basement membrane Glandular epithelium: serous, mucous and serous demilune glandular secretory units ELECTRON MICROSCOPY: Recognize types of epithelia (as above) and characteristic features of surface specializations that occur on apical, basal and lateral surfaces including the following: Apical surface: Microvilli, cilia, stereocilia, striated border, glycocalyx Basal surface: Hemidesmosomes and basal lamina Lateral surface: Cell junctions, zonula occludens, zonula adherens, macula adherens (desmosome), gap junction, glycocalyx ASSIGNMENT FOR TODAY’S LABORATORY GLASS SLIDES SL 63 (Heart with atrioventricular valve) simple squamous epithelium (lines heart) SL 137 (Ovary) Simple cuboidal epithelium (covers ovary) - varies in height SL 14 (Small intestine) Simple columnar epithelium SL 15B (Trachea) Pseudostratified columnar epithelium with goblet cells SL 156 (Epididymis) Pseudostratified -

Lecture7 Genmed 2Nd Semester
Lecture 7 GenMed_2nd semester Epithelial tissue – definition, classification and histogenesis Overview of covering and glandular epithelia. Characteristics of glandular cells Absorptive, respiratory, and sensory epithelia Epithelial tissue is composed of cells that are in close apposition with one another; among cells only a small amount of intercellular substance is present epithelial cells are usually of regular form without extensive cytoplasmic processes adhesion between cells is very strong epithelia derive from the all germ layers epithelial tissue is avascular - it contains no blood capillaries it exhibits a remarkable degree of physiologic regeneration Classification of the epithelial tissue a) according to the arrangement of cells -2 forms - epithelial membranes either sheets - composed of one or more cell layers in thickness or - solid cords or tubules (rarely follicles) that have developed as out- growths from an epithelial sheet cords and tubulus especially occur in glands b) according to the function of cells covering or protective - cells cover external and internal surfaces of human body and protect underlying tissues against loss moisture and mechanical damage, secretory or glandular - cells are engaged in synthetic processes and product substances with defined functional destination, absorptive - cells transport substances from the alimentary canal and renal tubules into the systemic circulation, respiratory - cells take part in the transport of oxygen and carbon dioxide from alveoli into the blood, sensory - cells are -

Oral Histology
Oral Histology Lec.17 Dr. Nada Al-Ghaban Salivary Glands: Salivary glands(S.G.) are exocrine,compound, acinar, either serous or mucous or mixed glands whose salivary secretions flow into the oral cavity .S.G. are classified as either major or minor depending on their size and the amount of their secretions. Major S.G. are three pairs of large glands-the parotid, submandibular, and sublingual glands, which located extra orally and carry their secretion some distance to the oral cavity by means of a main ducts. While the minor S.G. are numerous small glands widely distributed in the submucosa of oral cavity such as labial, lingual, palatal, buccal, glossopalatal and retromolar glands and these glands empty their products directly into the mouth by means of short ducts. Saliva is a complex fluid, produced by salivary glands, the most important function of which is to maintain the well-being of the mouth and moisture the oral cavity. The total volume of saliva secreted per day is about 1-1.5 liter in humans. Whole saliva or mixed saliva is referred to oral fluid which include the secretion of major and minor S.G., desquamated oral epith. cells, microorganism and their product, food debris and serum component and inflammatory cells that gain access in the gingival sulcus. Composition of saliva Saliva consists of 99% or more of water and 1% organic, the organic constituents of saliva are include: a.Enzymes(amylase,ribonucleas,kallikreins,esterase,hystatin,cysatin,peroxidase lysozymes lactoferin ,acid phosphatase) . b.Immunoglobulines (IgG and IgM). 1 c.Other factors( blood clotting factors ,amino acids, urea ,uric aacid,glucose) . -

Epithelia and Glands IUSM – 2016 I
Lab 4 – Epithelia and Glands IUSM – 2016 I. Introduction Epithelia and Glands II. Learning Objectives III. Keywords IV. Slides A. Epithelia 1. Simple a. Simple squamous b. Simple cuboidal c. Simple columnar d. Pseudostratified columnar 2. Stratified a. Stratified squamous b. Stratified cuboidal c. Stratified columnar d. Transitional B. Exocrine Glands 1. Simple (unbranched duct) a. Tubular b. Branched tubular c. Coiled tubular d. Branched acinar 2. Compound (branched ducts) a. Tubular b. Acinar c. Tubulo-acinar SEM of ciliated columnar epithelium of the uterine tube V. Summary Lab 4 – Epithelia and Glands IUSM – 2016 I. Introduction Epithelium II. Learning Objectives III. Keywords 1. Greek: epi – “upon”, thele – “teat, nipple” IV. Slides A. Epithelia 2. Avascular tissue that covers body surfaces, lines body cavities, and forms glands (endocrine and 1. Simple exocrine). a. Simple squamous b. Simple cuboidal 3. Composed of sheets of closely aggregated cells, of c. Simple columnar one or more layers thick, sitting upon a basement d. Pseudostratified columnar membrane. 2. Stratified 4. Creates a barrier between “external” environment a. Stratified squamous and underlying connective tissue. b. Stratified cuboidal c. Stratified columnar 5. Polarized with a free surface (apical surface), d. Transitional generally facing the external environment or lumen, B. Exocrine Glands and a bound surface (basal surface), facing the 1. Simple (unbranched duct) basement membrane. a. Tubular 6. Epithelial tissues are categorized by the number of b. Branched tubular cell-layers and the shape of their cells. c. Coiled tubular d. Branched acinar 7. Exocrine glands are categorized by the arrangement of their duct portion (branched or not) and the 2. -

Parotid Gland – Von Ebner’S Gland 2
Dental Histology 291 Salivary Gland Assist.Prof.Wacharaporn Thosaporn Dept. of Odontology & Oral Pathology Faculty of Dentistry Salivary gland Structure of gland 1. Development of salivary gland 2. Serous acini and zymogen granule 3. Mucous tubule or acini 4. Mixed acini – Mucous tubule or acini – Serous demilune 5. Duct 6. Capsule, septa 1 Salivary gland Type of gland 1. Pure serous gland – Parotid gland – von Ebner’s gland 2. Pure mucous gland – Palatine gland – Buccal gland – Weber’s gland Salivary gland Type of gland 3. Mixed gland, predominately with mucous type – Sublingual gland – Labial gland, anterior lingual gland 4. Mixed gland, predominately with serous type – Submandibular gland 2 Development of salivary gland Meckel’s cartilage Meckel’s cartilage Chondrocyte 3 Development of salivary gland Parenchyma Stroma Development of salivary gland Duct Acini Connective tissue 4 Parenchyma and stroma Duct Stroma Acini Duct Supporting connective tissue 5 Parenchyma and stroma Serous acini Septa Parenchyma Duct Serous acini 6 Septa Parenchyma Serous acini Duct 7 Parenchyma Serous acini Duct Saliva Duct 8 Serous acini Zymogen granule in Serous acini Parotid gland Duct Serous acini 9 Pure mucous gland (Buccal gland) Pure mucous gland (Buccal gland) 10 Pure mucous gland (Buccal gland) Pure mucous gland (mucicarmine stain) 11 Pure mucous gland (mucicarmine stain) Pure mucous gland (mucicarmine stain) 12 Pure mucous gland (mucicarmine stain) Mucicarmine stain (positive to mucin) 13 Parotid gland (pure serous) Capsule Septa Parotid gland Intralobular -

1 | Page: Epithelial Tissue Swailes Module 1.2: Epithelial Tissue N. Swailes, Ph.D. Department of Anatomy and Cell Biology Rm: B
Module 1.2: Epithelial Tissue N. Swailes, Ph.D. Department of Anatomy and Cell Biology Rm: B046A ML Tel: 5-7726 E-mail: [email protected] Recommended Reading Mescher AL, Junqueira’s Basic Histology Text and Atlas, 13th Edition, Chapter 4 (also via AccessMedicine) Learning Objectives 1) Describe the general characteristics of epithelial tissue 2) Determine that epithelial cells have polarity 3) Identify and explain the functions of: a. apical domain modifications (microvilli, cilia, stereocilia) b. lateral domain modifications (tight, adherens, gap and desmosome junctions) c. basal domain modifications (hemidesmosomes and basement membrane) 4) Employ criteria to fully classify the different types of epithelia in the body and, for each, suggest a function for the epithelium based on its morphology 5) Describe the epithelial origins of glands 6) Classify the different types of glands based upon their organization and secretion type Introduction The human body is made up of only four basic tissues: 1. Epithelial tissue 2. Connective tissue 3. Muscle tissue 4. Nervous tissue By adjusting the organization, composition and special features associated with each of these tissues it is possible to impart a wide variety of functions to the region or organ that they form. During this module you will examine the basic histological structure and function of Epithelial Tissue. You will learn to identify and fully classify this tissue and the glands derived from it. 1 | Page: Epithelial Tissue S w a i l e s Ectoderm: skin (epidermis) exterior oral cavity Part A: General Characteristics of Epithelial Tissues surfaces anal canal A1. An introduction to epithelia There are many classes but only two types of epithelia: i. -

Dr. Nemeskéri: Histology Manual
HOW TO USE THE HISTOLOGY MANUAL 1. You are expected to recognize only the structures highlighted in the text and to know the morphological and theoretical description of them. 2. Paragraphs in italics help you to identify the structures. Using italics, we draw your attention to pathologic processes occurring frequently in a given histological localization, which stresses the marked importance of precise knowledge of the structures involved. HOW TO STUDY THE HISTOLOGICAL SLIDES 1. Take the slide, and firstly study it with the naked eye (shape of the section, density of the tissue, colors etc.) 2. Place the slide into the microscope specimen holder ALWAYS with the COVER GLASS UPRIGHT!! 3. Always check the WHOLE SURFACE of the tissue section, starting with the lowest magnification followed by the medium magnification. Only change to the highest magnification when you have selected an area under the medium magnification objective lens. 4. NEVER start the identification of a slide with the highest magnification objective!!! 5. In the case when you are not able to identify the section, try to describe particular shapes of cells, number of cell layers, and staining properties of the cells. Check all of your statements in the microscope! 6. Do not try to illustrate all the structures seen in the round field of vision! Make a schematic drawing only of a part of the section which displays the most informative morphological details!! 7. Besides the recognition of histological structures, students are expected to answer theoretical questions related to structures in question (gross anatomy, embryology, ultrastructure, biochemistry). Your feedback on the Histology Manual is very important.