Comparative Functional Analysis of the Rac Gtpasesprovided by Elsevier - Publisher Connector
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Materials: Evaluation of Cytotoxicity and Α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Supplementary Materials: Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids Oxana B. Kazakova1*, Gul'nara V. Giniyatullina1, Akhat G. Mustafin1, Denis A. Babkov2, Elena V. Sokolova2, Alexander A. Spasov2* 1Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences, 71, pr. Oktyabrya, 450054 Ufa, Russian Federation 2Scientific Center for Innovative Drugs, Volgograd State Medical University, Novorossiyskaya st. 39, Volgograd 400087, Russian Federation Correspondence Prof. Dr. Oxana B. Kazakova Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences 71 Prospeсt Oktyabrya Ufa, 450054 Russian Federation E-mail: [email protected] Prof. Dr. Alexander A. Spasov Scientific Center for Innovative Drugs of the Volgograd State Medical University 39 Novorossiyskaya st. Volgograd, 400087 Russian Federation E-mail: [email protected] Figure S1. 1H and 13C of compound 2. H NH N H O H O H 2 2 Figure S2. 1H and 13C of compound 4. NH2 O H O H CH3 O O H H3C O H 4 3 Figure S3. Anticancer screening data of compound 2 at single dose assay 4 Figure S4. Anticancer screening data of compound 7 at single dose assay 5 Figure S5. Anticancer screening data of compound 8 at single dose assay 6 Figure S6. Anticancer screening data of compound 9 at single dose assay 7 Figure S7. Anticancer screening data of compound 12 at single dose assay 8 Figure S8. Anticancer screening data of compound 13 at single dose assay 9 Figure S9. Anticancer screening data of compound 14 at single dose assay 10 Figure S10. -

Active Rac1 Detection Kit 2012 09/20
Active Rac1 Detection Kit 1 Kit Orders n 877-616-CELL (2355) (30 immunoprecipitations) [email protected] Support n 877-678-TECH (8324) [email protected] Web n www.cellsignal.com rev. 09/24/20 #8815 For Research Use Only. Not For Use In Diagnostic Procedures. Species Cross-Reactivity: H, M Components Ship As: 11894S Item # Kit Quantity Storage Temp Description: The Active Rac1 Detection Kit provides all GTPgS 11521 1 X 50 µl –80°C reagents necessary for measuring activation of Rac1 GTPase in the cell. GST-PAK1-PBD fusion protein is used to bind GDP 11522 1 X 50 µl –80°C the activated form of GTP-bound Rac1, which can then be Components Ship As: 11894S Item # Kit Quantity Storage Temp immunoprecipitated with glutathione resin. Rac1 activation levels are then determined by western blot using a Rac1 GST-Human PAK1-PBD 8659 1 X 600 µg –20°C Mouse mAb. Rac1 Mouse mAb 8631 1 X 50 µl –20°C Specificity/Sensitivity: Active Rac1 Detection Kit detects Components Ship As: 11860S Item # Kit Quantity Storage Temp endogenous levels of GTP-bound (active) Rac1 as shown in Lysis/Binding/Wash Buffer 11524 1 X 100 mL 4°C Figure 1. This kit detects proteins from the indicated species, as determined through in-house testing, but may also detect Glutathione Resin 11523 1 X 3 ml 4°C homologous proteins from other species. SDS Sample Buffer 11525 1 X 1.5 ml 4°C Background: The Ras superfamily of small GTP-binding Spin Cup and Collection Tubes 11526 1 X 30 vial RT proteins (G proteins) comprise a large class of proteins (over 150 members) that can be classified into at least five families based on their sequence and functional similarities: 1 2 3 4 Figure 1. -

Identification of Pathogenic Genes of Pterygium Based on the Gene Expression Omnibus Database
Int J Ophthalmol, Vol. 12, No. 4, Apr.18, 2019 www.ijo.cn Tel: 8629-82245172 8629-82210956 Email: [email protected] ·Basic Research· Identification of pathogenic genes of pterygium based on the Gene Expression Omnibus database Xiao-Li Yue, Zi-Qing Gao Department of Ophthalmology, the First Affiliated Hospital of INTRODUCTION Bengbu Medical College, Bengbu 233000, Anhui Province, terygium is a general ocular surface and degenerative China P disease characterized by conjunctival fibrovascular Correspondence to: Xiao-Li Yue. Department of Ophthalmology, proliferation and invasion of the peripheral cornea. Pterygium the First Affiliated Hospital of Bengbu Medical College, is composed of the head that invades the cornea, the neck Bengbu Xinxin Jiayuan No.3 Building 2 unit 603, Bengbu that includes the superficial limbus and the body that overlie 233000, Anhui Province, China. [email protected] the sclera[1]. Diverse biological pathways including increase Received: 2018-02-06 Accepted: 2019-01-14 of mitotic proteins and protein implicated in tissue invasion were remarkably affected in pterygium. Additionally, Abstract fibrosis, inflammation, angiogenesis, collagen deposition ● AIM: To identify the pathogenic genes in pterygium. and extracellular matrix breakdown are associated with ● METHODS: We obtained mRNA expression profiles pterygium[2]. Pterygium is noted to be more prevalent than from the Gene Expression Omnibus database (GEO) to 20% of some populations and typically affects the younger identify differentially expressed genes -

Transcriptomic Analysis Reveals Abnormal Expression of Prion Disease Gene Pathway in Brains from Patients with Autism Spectrum Disorders
brain sciences Article Transcriptomic Analysis Reveals Abnormal Expression of Prion Disease Gene Pathway in Brains from Patients with Autism Spectrum Disorders Salvo Danilo Lombardo 1 , Giuseppe Battaglia 2,3, Maria Cristina Petralia 4, Katia Mangano 1 , Maria Sofia Basile 1, Valeria Bruno 2,3, Paolo Fagone 1,* , Rita Bella 5, Ferdinando Nicoletti 1 and Eugenio Cavalli 1 1 Department of Biomedical and Biotechnological Sciences, University of Catania, 95123 Catania, Italy; [email protected] (S.D.L.); [email protected] (K.M.); sofi[email protected] (M.S.B.); [email protected] (F.N.); [email protected] (E.C.) 2 Department of Physiology and Pharmacology, University Sapienza, Piazzale A. Moro, 5, 00185 Roma, Italy; [email protected] (G.B.); [email protected] (V.B.) 3 IRCCS Neuromed, Località Camerelle, 86077 Pozzilli, Italy 4 Department of Educational Sciences, University of Catania, 95124 Catania, Italy; [email protected] 5 Department of Medical Sciences, Surgery and Advanced Technologies, University of Catania, 95123 Catania, Italy; [email protected] * Correspondence: [email protected] Received: 26 February 2020; Accepted: 26 March 2020; Published: 29 March 2020 Abstract: The role of infections in the pathogenesis of autism spectrum disorder (ASD) is still controversial. In this study, we aimed to evaluate markers of infections and immune activation in ASD by performing a meta-analysis of publicly available whole-genome transcriptomic datasets of brain samples from autistic patients and otherwise normal people. Among the differentially expressed genes, no significant enrichment was observed for infectious diseases previously associated with ASD, including herpes simplex virus-1 (HSV-1), cytomegalovirus and Epstein–Barr virus in brain samples, nor was it found in peripheral blood from ASD patients. -

Pig Antibodies
Pig Antibodies gene_name sku Entry_Name Protein_Names Organism Length Identity CDX‐2 ARP31476_P050 D0V4H7_PIG Caudal type homeobox 2 (Fragment) Sus scrofa (Pig) 147 100.00% CDX‐2 ARP31476_P050 A7MAE3_PIG Caudal type homeobox transcription factor 2 (Fragment) Sus scrofa (Pig) 75 100.00% Tnnt3 ARP51286_P050 Q75NH3_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 271 85.00% Tnnt3 ARP51286_P050 Q75NH2_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 266 85.00% Tnnt3 ARP51286_P050 Q75NH1_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 260 85.00% Tnnt3 ARP51286_P050 Q75NH0_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 250 85.00% Tnnt3 ARP51286_P050 Q75NG8_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 266 85.00% Tnnt3 ARP51286_P050 Q75NG7_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 260 85.00% Tnnt3 ARP51286_P050 Q75NG6_PIG Troponin T fast skeletal muscle type Sus scrofa (Pig) 250 85.00% Tnnt3 ARP51286_P050 TNNT3_PIG Troponin T, fast skeletal muscle (TnTf) Sus scrofa (Pig) 271 85.00% ORF Names:PANDA_000462 EMBL EFB13877.1OrganismAiluropod High mobility group protein B2 (High mobility group protein a melanoleuca (Giant panda) ARP31939_P050 HMGB2_PIG 2) (HMG‐2) Sus scrofa (Pig) 210 100.00% Agpat5 ARP47429_P050 B8XTR3_PIG 1‐acylglycerol‐3‐phosphate O‐acyltransferase 5 Sus scrofa (Pig) 365 85.00% irf9 ARP31200_P050 Q29390_PIG Transcriptional regulator ISGF3 gamma subunit (Fragment) Sus scrofa (Pig) 57 100.00% irf9 ARP31200_P050 Q0GFA1_PIG Interferon regulatory factor 9 Sus scrofa (Pig) -

Supplemental Digital Content (Sdc) Sdc, Materials
SUPPLEMENTAL DIGITAL CONTENT (SDC) SDC, MATERIALS AND METHODS Animals This study used 9-12 week old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). This study conformed to the National Institutes of Health guidelines and was conducted under animal protocols approved by the University of Virginia’s Institutional Animal Care and Use Committee. Murine DCD Lung Procedure Mice were anesthetized by isoflurane inhalation and euthanized by cervical dislocation followed by a 60-minute period of “no-touch” warm ischemia. Mice then underwent extended median sternotomy and midline cervical exposure followed by intubation for the initiation of mechanical ventilation at 120 strokes/minute with room air. The left atrium was vented via an atriotomy followed by infusion of the lungs with 3 mL 4°C Perfadex® solution (Vitrolife Inc., Denver, CO) supplemented with THAM Solution (Vitrolife, Kungsbacka, Sweden), estimating weight-based volume recommendations for pulmonary artery perfusion (140mL/kg) (1). The chest was then packed with ice and the trachea occluded by silk-suture tie at tidal volume (7µL/g body weight) prior to cold static preservation (CSP) for 60 minutes at 4°C. Mice were then randomized into three experimental groups: 1) CSP alone with no EVLP, 2) EVLP with Steen solution and 3) EVLP with Steen solution supplemented with the highly selective A2AR agonist, ATL1223 (30nM, Lewis and Clark Pharmaceuticals, Charlottesville, VA). Mice treated with ATL1223 during EVLP also received ATL1223 treatment (30nM) during the Perfadex flush prior to CSP whereas the EVLP group received vehicle (DMSO) during the flush. CSP lungs, which did not undergo EVLP, underwent immediate functional assessment after re-intubation as described below. -
Drosophila and Human Transcriptomic Data Mining Provides Evidence for Therapeutic
Drosophila and human transcriptomic data mining provides evidence for therapeutic mechanism of pentylenetetrazole in Down syndrome Author Abhay Sharma Institute of Genomics and Integrative Biology Council of Scientific and Industrial Research Delhi University Campus, Mall Road Delhi 110007, India Tel: +91-11-27666156, Fax: +91-11-27662407 Email: [email protected] Nature Precedings : hdl:10101/npre.2010.4330.1 Posted 5 Apr 2010 Running head: Pentylenetetrazole mechanism in Down syndrome 1 Abstract Pentylenetetrazole (PTZ) has recently been found to ameliorate cognitive impairment in rodent models of Down syndrome (DS). The mechanism underlying PTZ’s therapeutic effect is however not clear. Microarray profiling has previously reported differential expression of genes in DS. No mammalian transcriptomic data on PTZ treatment however exists. Nevertheless, a Drosophila model inspired by rodent models of PTZ induced kindling plasticity has recently been described. Microarray profiling has shown PTZ’s downregulatory effect on gene expression in fly heads. In a comparative transcriptomics approach, I have analyzed the available microarray data in order to identify potential mechanism of PTZ action in DS. I find that transcriptomic correlates of chronic PTZ in Drosophila and DS counteract each other. A significant enrichment is observed between PTZ downregulated and DS upregulated genes, and a significant depletion between PTZ downregulated and DS dowwnregulated genes. Further, the common genes in PTZ Nature Precedings : hdl:10101/npre.2010.4330.1 Posted 5 Apr 2010 downregulated and DS upregulated sets show enrichment for MAP kinase pathway. My analysis suggests that downregulation of MAP kinase pathway may mediate therapeutic effect of PTZ in DS. Existing evidence implicating MAP kinase pathway in DS supports this observation. -

The Rac3 Gtpase in Neuronal Development, Neurodevelopmental Disorders, and Cancer
cells Review The Rac3 GTPase in Neuronal Development, Neurodevelopmental Disorders, and Cancer Ivan de Curtis Cell Adhesion Unit, Division of Neuroscience, San Raffaele Scientific Institute and Vita-Salute San Raffaele University, 20132 Milano, Italy; [email protected] Received: 27 August 2019; Accepted: 8 September 2019; Published: 11 September 2019 Abstract: Rho family small guanosine triphosphatases (GTPases) are important regulators of the cytoskeleton, and are critical in many aspects of cellular and developmental biology, as well as in pathological processes such as intellectual disability and cancer. Of the three members of the family, Rac3 has a more restricted expression in normal tissues compared to the ubiquitous member of the family, Rac1. The Rac3 polypeptide is highly similar to Rac1, and orthologues of the gene for Rac3 have been found only in vertebrates, indicating the late appearance of this gene during evolution. Increasing evidence over the past few years indicates that Rac3 plays an important role in neuronal development and in tumor progression, with specificities that distinguish the functions of Rac3 from the established functions of Rac1 in these processes. Here, results highlighting the importance of Rac3 in distinct aspects of neuronal development and tumor cell biology are presented, in support of the non-redundant role of different members of the two Rac GTPases in physiological and pathological processes. Keywords: Rho family GTPases; Rac3; neuronal development; mutations; intellectual disability; cancer; invasion; metastasis 1. Introduction In vertebrates the Rac family of guanosine triphosphatases (GTPases) includes three different genes that encode three highly similar proteins: the ubiquitously expressed Rac1 with its alternative spliced variant Rac1b [1,2]; Rac2 that is expressed only in hematopoietic cells [3]; and the Rac3 protein that is co-expressed with Rac1 in developing neurons and in other cell types, with a pattern of expression more restricted compared to Rac1 [4,5]. -
Oligodendrocytes Remodel the Genomic Fabrics of Functional Pathways in Astrocytes
1 Article 2 Oligodendrocytes remodel the genomic fabrics of 3 functional pathways in astrocytes 4 Dumitru A Iacobas 1,2,*, Sanda Iacobas 3, Randy F Stout 4 and David C Spray 2,5 5 Supplementary Material 6 Table S1. Genes whose >1.5x absolute fold-change did not meet the individual CUT criterion. 7 Red/green background of the expression ratio indicates not significant (false) up-/down-regulation. Gene Description X CUT Acap2 ArfGAP with coiled-coil, ankyrin repeat and PH domains 2 -1.540 1.816 Adamts18 a disintegrin-like and metallopeptidase -1.514 1.594 Akr1c12 aldo-keto reductase family 1, member C12 1.866 1.994 Alx3 aristaless-like homeobox 3 1.536 1.769 Alyref2 Aly/REF export factor 2 -1.880 2.208 Ankrd33b ankyrin repeat domain 33B 1.593 1.829 Ankrd45 ankyrin repeat domain 45 1.514 1.984 Ankrd50 ankyrin repeat domain 50 1.628 1.832 Ankrd61 ankyrin repeat domain 61 1.645 1.802 Arid1a AT rich interactive domain 1A -1.668 2.066 Artn artemin 1.524 1.732 Aspm abnormal spindle microtubule assembly -1.693 1.716 Atp6v1e1 ATPase, H+ transporting, lysosomal V1 subunit E1 -1.679 1.777 Bag4 BCL2-associated athanogene 4 1.723 1.914 Birc3 baculoviral IAP repeat-containing 3 -1.588 1.722 Ccdc104 coiled-coil domain containing 104 -1.819 2.130 Ccl2 chemokine -1.699 2.034 Cdc20b cell division cycle 20 homolog B 1.512 1.605 Cenpf centromere protein F 2.041 2.128 Cep97 centrosomal protein 97 -1.641 1.723 COX1 mitochondrially encoded cytochrome c oxidase I -1.607 1.650 Cpsf7 cleavage and polyadenylation specific factor 7 -1.635 1.891 Crct1 cysteine-rich -

Translational Profiling Reveals the Transcriptome of Leptin Receptor Neurons and Its Regulation by Leptin
TRANSLATIONAL PROFILING REVEALS THE TRANSCRIPTOME OF LEPTIN RECEPTOR NEURONS AND ITS REGULATION BY LEPTIN by Margaret B. Allison A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Molecular and Integrative Physiology) In the University of Michigan 2015 Doctoral Committee: Professor Martin G. Myers Jr., Chair Associate Professor Carol F. Elias Professor Malcolm J. Low Professor Suzanne Moenter Professor Audrey Seasholtz Before you leave these portals To meet less fortunate mortals There's just one final message I would give to you: You all have learned reliance On the sacred teachings of science So I hope, through life, you never will decline In spite of philistine defiance To do what all good scientists do: Experiment! -- Cole Porter There is no cure for curiosity. -- unknown © Margaret Brewster Allison 2015 ACKNOWLEDGEMENTS If it takes a village to raise a child, it takes a research university to raise a graduate student. There are many people who have supported me over the past six years at Michigan, and it is hard to imagine pursuing my PhD without them. First and foremost among all the people I need to thank is my mentor, Martin. Nothing I might say here would ever suffice to cover the depth and breadth of my gratitude to him. Without his patience, his insight, and his at times insufferably positive outlook, I don’t know where I would be today. Martin supported my intellectual curiosity, honed my scientific inquiry, and allowed me to do some really fun research in his lab. It was a privilege and a pleasure to work for him and with him. -

Rac3-Mediated Transformation Requires Multiple Effector Pathways
Research Article Rac3-Mediated Transformation Requires Multiple Effector Pathways Patricia J. Keller,1 Christyn M. Gable,2 Michele R. Wing,1 and Adrienne D. Cox1,2,3,4 Departments of 1Pharmacology, 2Biology, and 3Radiation Oncology, and 4Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina Abstract development and progression of cancer (5). Rac1 is essential for progression of the cell cycle and activates several pathways known to Our initial characterization of Rac3, a close relative of the small GTPase Rac1, established its ability to promote membrane be important in cellular proliferation, such as serum response factor (SRF), cyclin D1, and E2F (6, 7). Rac1 also promotes cellular survival ruffling, transformation, and activation of c-jun transcription- n al activity. The finding that Rac3 is transforming, and its by activating the nuclear factor- B pathway and by preventing similarity to Rac1, a protein that has a well-established anoikis and apoptosis (8–10). Not surprisingly, due to its role in connection to many processes important for cancer progres- promoting cell cycle progression and survival, Rac1 also promotes sion, prompted further investigation into Rac3 transformation. transformation in rodent fibroblast models and is required for We used effector domain mutants (EDMs) to explore the transformation induced by Ras (11–14). Due to its role in modulating relationship among Rac signaling, transformation, and effector the actin cytoskeleton, Rac1 also promotes motility and invasion, usage. All Rac3 EDMs tested (N26D, F37L, Y40C, and N43D) activities that are important for tumor metastasis (15). Given these retained the ability to promote membrane ruffling and focus activities, Rac1 has become a target for inhibitors that may be useful formation. -
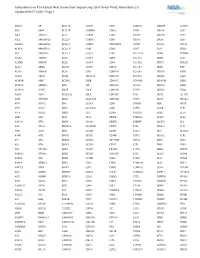
Comprehensive Pan-Cancer Next Generation Sequencing Solid Tumor Panel, Aberration List Updated 08-07-2020 --Page 1
Comprehensive Pan-Cancer Next Generation Sequencing Solid Tumor Panel, Aberration List Updated 08-07-2020 --Page 1 ABCC3 AR BCL11A CANT1 CDK1 CMKLR1 DAB2IP DUSP9 ABI1 ARAF BCL11B CAPRIN1 CDK12 CNBP DACH1 E2F1 ABL1 ARFRP1 BCL2 CAPZB CDK2 CNOT2 DACH2 E2F3 ABL2 ARHGAP20 BCL2A1 CARD11 CDK4 CNTN1 DAXX EBF1 ABLIM1 ARHGAP26 BCL2L1 CARM1 CDK5RAP2 CNTRL DCLK2 ECT2L ACACA ARHGEF12 BCL2L11 CARS CDK6 COG5 DCN EDIL3 ACE ARHGEF7 BCL2L2 CASC5 CDK7 COL11A1 DDB1 EDNRB ACER1 ARID1A BCL3 CASP3 CDK8 COL1A1 DDB2 EED ACSBG1 ARID1B BCL6 CASP7 CDK9 COL1A2 DDIT3 EEFSEC ACSL3 ARID2 BCL7A CASP8 CDKL5 COL3A1 DDR2 EGF ACSL6 ARID5B BCL9 CAV1 CDKN1A COL6A3 DDX10 EGFR ACVR1 ARIH2 BCOR CBFA2T3 CDKN1B COL9A3 DDX20 EGR1 ACVR1B ARNT BCORL1 CBFB CDKN1C COMMD1 DDX39B EGR2 ACVR1C ARRDC4 BCR CBL CDKN2A COX6C DDX3X EGR3 ACVR2A ASMTL BDNF CBLB CDKN2B CPNE1 DDX41 EGR4 ADD3 ASPH BHLHE22 CBLC CDKN2C CPS1 DDX5 EIF1AX ADM ASPSCR1 BICC1 CCDC28A CDKN2D CPSF6 DDX6 EIF4A2 AFF1 ASTN2 BIN1 CCDC6 CDX1 CRADD DEK EIF4E AFF3 ASXL1 BIRC3 CCDC88C CDX2 CREB1 DGKB ELF3 AFF4 ASXL2 BIRC6 CCK CEBPA CREB3L1 DGKI ELF4 AGR3 ATF1 BLM CCL2 CEBPB CREB3L2 DGKZ ELK4 AHCYL1 ATF3 BMP4 CCNA2 CEBPD CREBBP DICER1 ELL AHI1 ATG13 BMPR1A CCNB1IP1 CEBPE CRKL DIRAS3 ELN AHR ATG5 BRAF CCNB3 CENPF CRLF2 DIS3 ELOVL2 AHRR ATIC BRCA1 CCND1 CENPU CRTC1 DIS3L2 ELP2 AIP ATL1 BRCA2 CCND2 CEP170B CRTC3 DKK1 EML1 AK2 ATM BRCC3 CCND3 CEP57 CSF1 DKK2 EML4 AK5 ATP1B4 BRD1 CCNE1 CEP85L CSF1R DKK4 ENPP2 AKAP12 ATP8A2 BRD3 CCNG1 CHCHD7 CSF3 DLEC1 EP300 AKAP6 ATR BRD4 CCT6B CHD2 CSF3R DLL1 EP400 AKAP9 ATRNL1 BRIP1 CD19 CHD4 CSNK1A1 DLL3