The RHO Family Gtpases: Mechanisms of Regulation and Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Therapeutic Effects of Rho-ROCK Inhibitors on CNS Disorders
REVIEW The therapeutic effects of Rho-ROCK inhibitors on CNS disorders Takekazu Kubo1 Abstract: Rho-kinase (ROCK) is a serine/threonine kinase and one of the major downstream Atsushi Yamaguchi1 effectors of the small GTPase Rho. The Rho-ROCK pathway is involved in many aspects of Nobuyoshi Iwata2 neuronal functions including neurite outgrowth and retraction. The Rho-ROCK pathway becomes Toshihide Yamashita1,3 an attractive target for the development of drugs for treating central nervous system (CNS) dis- orders, since it has been recently revealed that this pathway is closely related to the pathogenesis 1Department of Neurobiology, Graduate School of Medicine, Chiba of several CNS disorders such as spinal cord injuries, stroke, and Alzheimer’s disease (AD). In University, 1-8-1 Inohana, Chuo-ku, the adult CNS, injured axons regenerate poorly due to the presence of myelin-associated axonal 2 Chiba 260-8670, Japan; Information growth inhibitors such as myelin-associated glycoprotein (MAG), Nogo, oligodendrocyte- Institute for Medical Research Ltd.; 3Department of Molecular myelin glycoprotein (OMgp), and the recently identifi ed repulsive guidance molecule (RGM). Neuroscience, Graduate School The effects of these inhibitors are reversed by blockade of the Rho-ROCK pathway in vitro, of Medicine, Osaka University 2-2 and the inhibition of this pathway promotes axonal regeneration and functional recovery in the Yamadaoka, Suita, Osaka 565-0871, Japan injured CNS in vivo. In addition, the therapeutic effects of the Rho-ROCK inhibitors have been demonstrated in animal models of stroke. In this review, we summarize the involvement of the Rho-ROCK pathway in CNS disorders such as spinal cord injuries, stroke, and AD and also discuss the potential of Rho-ROCK inhibitors in the treatment of human CNS disorders. -

Supplementary Materials: Evaluation of Cytotoxicity and Α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Supplementary Materials: Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids Oxana B. Kazakova1*, Gul'nara V. Giniyatullina1, Akhat G. Mustafin1, Denis A. Babkov2, Elena V. Sokolova2, Alexander A. Spasov2* 1Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences, 71, pr. Oktyabrya, 450054 Ufa, Russian Federation 2Scientific Center for Innovative Drugs, Volgograd State Medical University, Novorossiyskaya st. 39, Volgograd 400087, Russian Federation Correspondence Prof. Dr. Oxana B. Kazakova Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences 71 Prospeсt Oktyabrya Ufa, 450054 Russian Federation E-mail: [email protected] Prof. Dr. Alexander A. Spasov Scientific Center for Innovative Drugs of the Volgograd State Medical University 39 Novorossiyskaya st. Volgograd, 400087 Russian Federation E-mail: [email protected] Figure S1. 1H and 13C of compound 2. H NH N H O H O H 2 2 Figure S2. 1H and 13C of compound 4. NH2 O H O H CH3 O O H H3C O H 4 3 Figure S3. Anticancer screening data of compound 2 at single dose assay 4 Figure S4. Anticancer screening data of compound 7 at single dose assay 5 Figure S5. Anticancer screening data of compound 8 at single dose assay 6 Figure S6. Anticancer screening data of compound 9 at single dose assay 7 Figure S7. Anticancer screening data of compound 12 at single dose assay 8 Figure S8. Anticancer screening data of compound 13 at single dose assay 9 Figure S9. Anticancer screening data of compound 14 at single dose assay 10 Figure S10. -

Structure of the Dock2âˆ'elmo1 Complex Provides Insights Into
ARTICLE https://doi.org/10.1038/s41467-020-17271-9 OPEN Structure of the DOCK2−ELMO1 complex provides insights into regulation of the auto-inhibited state Leifu Chang1,7, Jing Yang1, Chang Hwa Jo 2, Andreas Boland 1,8, Ziguo Zhang 1, Stephen H. McLaughlin 1, Afnan Abu-Thuraia 3, Ryan C. Killoran2, Matthew J. Smith 2,4,9, Jean-Francois Côté 3,5,6,9 & ✉ David Barford 1 DOCK (dedicator of cytokinesis) proteins are multidomain guanine nucleotide exchange 1234567890():,; factors (GEFs) for RHO GTPases that regulate intracellular actin dynamics. DOCK proteins share catalytic (DOCKDHR2) and membrane-associated (DOCKDHR1) domains. The structurally-related DOCK1 and DOCK2 GEFs are specific for RAC, and require ELMO (engulfment and cell motility) proteins for function. The N-terminal RAS-binding domain (RBD) of ELMO (ELMORBD) interacts with RHOG to modulate DOCK1/2 activity. Here, we determine the cryo-EM structures of DOCK2−ELMO1 alone, and as a ternary complex with RAC1, together with the crystal structure of a RHOG−ELMO2RBD complex. The binary DOCK2−ELMO1 complex adopts a closed, auto-inhibited conformation. Relief of auto- inhibition to an active, open state, due to a conformational change of the ELMO1 subunit, exposes binding sites for RAC1 on DOCK2DHR2, and RHOG and BAI GPCRs on ELMO1. Our structure explains how up-stream effectors, including DOCK2 and ELMO1 phosphorylation, destabilise the auto-inhibited state to promote an active GEF. 1 MRC Laboratory of Molecular Biology, Cambridge CB2 0QH, UK. 2 Institute for Research in Immunology and Cancer, Université de Montréal, Montréal, Québec H3T 1J4, Canada. 3 Montreal Institute of Clinical Research (IRCM), Montréal, QC H2W 1R7, Canada. -

Active Rac1 Detection Kit 2012 09/20
Active Rac1 Detection Kit 1 Kit Orders n 877-616-CELL (2355) (30 immunoprecipitations) [email protected] Support n 877-678-TECH (8324) [email protected] Web n www.cellsignal.com rev. 09/24/20 #8815 For Research Use Only. Not For Use In Diagnostic Procedures. Species Cross-Reactivity: H, M Components Ship As: 11894S Item # Kit Quantity Storage Temp Description: The Active Rac1 Detection Kit provides all GTPgS 11521 1 X 50 µl –80°C reagents necessary for measuring activation of Rac1 GTPase in the cell. GST-PAK1-PBD fusion protein is used to bind GDP 11522 1 X 50 µl –80°C the activated form of GTP-bound Rac1, which can then be Components Ship As: 11894S Item # Kit Quantity Storage Temp immunoprecipitated with glutathione resin. Rac1 activation levels are then determined by western blot using a Rac1 GST-Human PAK1-PBD 8659 1 X 600 µg –20°C Mouse mAb. Rac1 Mouse mAb 8631 1 X 50 µl –20°C Specificity/Sensitivity: Active Rac1 Detection Kit detects Components Ship As: 11860S Item # Kit Quantity Storage Temp endogenous levels of GTP-bound (active) Rac1 as shown in Lysis/Binding/Wash Buffer 11524 1 X 100 mL 4°C Figure 1. This kit detects proteins from the indicated species, as determined through in-house testing, but may also detect Glutathione Resin 11523 1 X 3 ml 4°C homologous proteins from other species. SDS Sample Buffer 11525 1 X 1.5 ml 4°C Background: The Ras superfamily of small GTP-binding Spin Cup and Collection Tubes 11526 1 X 30 vial RT proteins (G proteins) comprise a large class of proteins (over 150 members) that can be classified into at least five families based on their sequence and functional similarities: 1 2 3 4 Figure 1. -
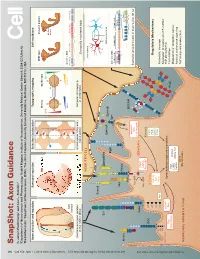
Snapshot: Axon Guidance Pasterkamp R
494 1 Cell Cell ??? SnapShot: Axon Guidance 153 SnapShot: XXXXXXXXXXXXXXXXXXXXXXXXXX 1 2 , ??MONTH?? ??DATE??, 200? ©200? Elsevier Inc. 200?©200? ElsevierInc. , ??MONTH?? ??DATE??, DOI R. Jeroen Pasterkamp and Alex L. Kolodkin , April11, 2013©2013Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.03.031 AUTHOR XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX 1 AFFILIATIONDepartment of XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Neuroscience and Pharmacology, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, 3584 CG Utrecht, The Netherlands; 2Department of Neuroscience, HHMI, The Johns Hopkins University School of Medicine, Baltimore, MD 21212, USA Axon attraction and repulsion Surround repulsion Selective fasciculation Topographic mapping Self-avoidance Wild-type Dscam1 mutant Sema3A A Retina SC/Tectum EphB EphrinB P D D Mutant T A neuron N P A V V Genomic DNA P EphA EphrinA Slit Exon 4 (12) Exon 6 (48) Exon 9 (33) Exon 17 (2) Netrin Commissural axon guidance Surround repulsion of peripheral Grasshopper CNS axon Retinotectal mapping at the CNS midline nerves in vertebrates fasciculation in vertebrates Drosophila mushroom body XXXXXXXXX NEURITE/CELL Isoneuronal Sema3 Slit Sema1/4-6 Heteroneuronal EphrinA EphrinB FasII Eph Genomic DNA Pcdh-α (14) Pcdh-β (22) Pcdh-γ (22) Variable Con Variable Con Nrp Plexin ** *** Netrin LAMELLIPODIA Con DSCAM ephexin Starburst amacrine cells in mammalian retina Ras-GTP Vav See online version for legend and references. α-chimaerin GEFs/GAPs Robo FARP Ras-GDP LARG RhoGEF Kinases DCC cc0 GTPases PKA cc1 FAK Regulatory Mechanisms See online versionfor??????. Cdc42 GSK3 cc2 Rac PI3K P1 Rho P2 cc3 Abl Proteolytic cleavage P3 Regulation of expression (TF, miRNA, FILOPODIA srGAP Cytoskeleton regulatory proteins multiple isoforms) Sos Trio Pcdh Cis inhibition DOCK180 PAK ROCK Modulation of receptors’ output LIMK Myosin-II Colin Forward and reverse signaling Actin Trafcking and endocytosis NEURONAL GROWTH CONE Microtubules SnapShot: Axon Guidance R. -

Rac1 Accumulates in the Nucleus During the G2 Phase of the Cell
Published Online: 28 April, 2008 | Supp Info: http://doi.org/10.1083/jcb.200801047 JCB: ARTICLE Downloaded from jcb.rupress.org on May 9, 2018 Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division David Michaelson , 2,5 Wasif Abidi , 2,5 Daniele Guardavaccaro , 4,5 Mo Zhou, 3,5 Ian Ahearn , 3,5 Michele Pagano , 4,5 and Mark R. Philips 1,2,3,5 1 Department of Medicine, 2 Department of Cell Biology, 3 Department of Pharmacology, 4 Department of Pathology, and the 5 New York University Cancer Institute, New York University School of Medicine, New York, NY 10016 ac1 regulates a wide variety of cellular processes. zation of cells stably expressing low levels of GFP-Rac1, The polybasic region of the Rac1 C terminus func- and time-lapse microscopy of asynchronous cells revealed Rtions both as a plasma membrane – targeting motif Rac1 accumulation in the nucleus in late G2 and exclu- and a nuclear localization sequence (NLS). We show that sion in early G1. Although constitutively active Rac1 re- a triproline N-terminal to the polybasic region contributes stricted to the cytoplasm inhibited cell division, activated to the NLS, which is cryptic in the sense that it is strongly Rac1 expressed constitutively in the nucleus increased the inhibited by geranylgeranylation of the adjacent cysteine. mitotic rate. These results show that Rac1 cycles in and out Subcellular fractionation demonstrated endogenous Rac1 of the nucleus during the cell cycle and thereby plays a in the nucleus and Triton X-114 partition revealed that this role in promoting cell division. -

Ran Activation Assay Kit
Product Manual Ran Activation Assay Kit Catalog Number STA-409 20 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Small GTP-binding proteins (or GTPases) are a family of proteins that serve as molecular regulators in signaling transduction pathways. Ran, a 25 kDa protein of the Ras superfamily, regulates a variety of biological response pathways that include DNA synthesis, cell cycle progression, and translocation of RNA/proteins through the nuclear pore complex. Like other small GTPases, Ran regulates molecular events by cycling between an inactive GDP-bound form and an active GTP-bound form. In its active (GTP-bound) state, Ran binds specifically to RanBP1 to control downstream signaling cascades. Cell Biolabs’ Ran Activation Assay Kit utilizes RanBP1 Agarose beads to selectively isolate and pull- down the active form of Ran from purified samples or endogenous lysates. Subsequently, the precipitated GTP-Ran is detected by western blot analysis using an anti-Ran antibody. Cell Biolabs’ Ran Activation Assay Kit provides a simple and fast tool to monitor the activation of Ran. The kit includes easily identifiable RanBP1 Agarose beads (see Figure 1), pink in color, and a GTPase Immunoblot Positive Control for quick Ran identification. Each kit provides sufficient quantities to perform 20 assays. Figure 1: RanBP1 Agarose beads, in color, are easy to visualize, minimizing potential loss during washes and aspirations. 2 Assay Principle Related Products 1. STA-400: Pan-Ras Activation Assay Kit 2. STA-400-H: H-Ras Activation Assay Kit 3. STA-400-K: K-Ras Activation Assay Kit 4. STA-400-N: N-Ras Activation Assay Kit 5. -

Pi3kp110-, Src-, FAK-Dependent and DOCK2-Independent Migration and Invasion of CXCL13-Stimulated Prostate Cancer Cells
El Haibi et al. Molecular Cancer 2010, 9:85 http://www.molecular-cancer.com/content/9/1/85 RESEARCH Open Access PI3Kp110-,Research Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells Christelle P El Haibi1, Praveen K Sharma2, Rajesh Singh3, Paul R Johnson4, Jill Suttles2, Shailesh Singh3 and James W Lillard Jr*3 Abstract Background: Most prostate cancer (PCa)-related deaths are due to metastasis, which is mediated in part by chemokine receptor and corresponding ligand interaction. We have previously shown that PCa tissue and cell lines express high levels of the chemokine receptor CXCR5, than compared to their normal counterparts, and interaction of CXCR5 with its specific ligand (CXCL13) promoted PCa cell invasion, migration, and differential matrix metalloproteinase (MMP) expression. This study dissects some of the molecular mechanisms following CXCL13-CXCR5 interaction that mediate PCa cell migration and invasion. Results: Using Western blot analysis, kinase-specific cell-based ELISAs, and migration and invasion assays, we show that PCa cell lines differentially express phosphoinositide-3 kinase (PI3K) catalytic subunit isoforms and dedicator of cytokinesis 2 (DOCK2). Specifically, we show that PC3 and normal prostatic epithelial (RWPE-1), but not LNCaP cell lines expressed DOCK2, while RWPE, PC3, and LNCaP cell lines expressed PI3K-p110α and -p110β. Moreover, PC3 selectively expressed PI3K-p110γ, but LNCaP and RWPE cell lines expressed PI3Kp110δ. CXCL13 caused CXCR5-dependent activation of the PI3Kp85α in LNCaP cells, and p85α as well as -p101 in PC3 cells. CXCL13-CXCR5 interaction regulated LNCaP and PC3 cell migration and invasion through extracellular signal-regulated kinase 1/2 (ERK1/2) activation that was primarily dependent on the PI3Kp110 isoform(s), Src, and focal adhesion kinase (FAK), but not DOCK2. -

Characterization of Gf a Drosophila Trimeric G Protein Alpha Subunit
Characterization of Gf a Drosophila trimeric G protein alpha subunit Naureen Quibria Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2012 © 2012 Naureen Quibria All rights reserved Abstract Characterization of Gf a Drosophila trimeric G-protein alpha subunit Naureen Quibria In the morphogenesis of tissue development, how coordination of patterning and growth achieve the correct organ size and shape is a principal question in biology. Efficient orchestrating mechanisms are required to achieve this and cells have developed sophisticated systems for reception and interpretation of the multitude of extracellular stimuli to which they are exposed. Plasma membrane receptors play a key role in the transmission of such signals. G-protein coupled receptors (GPCRs) are the largest class of cell surface receptors that respond to an enormous diversity of extracellular stimuli, and are critical mediators of cellular signal transduction in eukaryotic organisms. Signaling through GPCRs has been well characterized in many biological contexts. While they are a major class of signal transducers, there are not many defined instances where GPCRs have been implicated in the process of development to date. The Drosophila wing provides an ideal model system to elucidate and address the role of GPCRs in development, as its growth is regulated by a small number of conserved signaling pathways. In my thesis work, I address the role of a trimeric G alpha protein in Drosophila, Gαf, and what part it may play in development. In particular, I explore the role of Gαf as an alpha subunit of a trimeric complex, to determine what heptahelical receptors might act as its cognate receptor. -

The Rac Gtpase in Cancer: from Old Concepts to New Paradigms Marcelo G
Published OnlineFirst August 14, 2017; DOI: 10.1158/0008-5472.CAN-17-1456 Cancer Review Research The Rac GTPase in Cancer: From Old Concepts to New Paradigms Marcelo G. Kazanietz1 and Maria J. Caloca2 Abstract Rho family GTPases are critical regulators of cellular func- mislocalization of Rac signaling components. The unexpected tions that play important roles in cancer progression. Aberrant pro-oncogenic functions of Rac GTPase-activating proteins also activity of Rho small G-proteins, particularly Rac1 and their challenged the dogma that these negative Rac regulators solely regulators, is a hallmark of cancer and contributes to the act as tumor suppressors. The potential contribution of Rac tumorigenic and metastatic phenotypes of cancer cells. This hyperactivation to resistance to anticancer agents, including review examines the multiple mechanisms leading to Rac1 targeted therapies, as well as to the suppression of antitumor hyperactivation, particularly focusing on emerging paradigms immune response, highlights the critical need to develop ther- that involve gain-of-function mutations in Rac and guanine apeutic strategies to target the Rac pathway in a clinical setting. nucleotide exchange factors, defects in Rac1 degradation, and Cancer Res; 77(20); 5445–51. Ó2017 AACR. Introduction directed toward targeting Rho-regulated pathways for battling cancer. Exactly 25 years ago, two seminal papers by Alan Hall and Nearly all Rho GTPases act as molecular switches that cycle colleagues illuminated us with one of the most influential dis- between GDP-bound (inactive) and GTP-bound (active) forms. coveries in cancer signaling: the association of Ras-related small Activation is promoted by guanine nucleotide exchange factors GTPases of the Rho family with actin cytoskeleton reorganization (GEF) responsible for GDP dissociation, a process that normally (1, 2). -

Regulation of the Mammalian Target of Rapamycin Complex 2 (Mtorc2)
Regulation of the Mammalian Target Of Rapamycin Complex 2 (mTORC2) Inauguraldissertation Zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Klaus-Dieter Molle aus Heilbronn, Deutschland Basel, 2006 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät Auf Antrag von Prof. Michael N. Hall und Prof. Markus Affolter. Basel, den 21.11.2006 Prof. Hans-Peter Hauri Dekan Summary The growth controlling mammalian Target of Rapamycin (mTOR) is a conserved Ser/Thr kinase found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. The tumor suppressor TSC1-TSC2 complex inhibits mTORC1 by acting on the small GTPase Rheb, but the role of TSC1-TSC2 and Rheb in the regulation of mTORC2 is unclear. Here we examined the role of TSC1-TSC2 in the regulation of mTORC2 in human embryonic kidney 293 cells. Induced knockdown of TSC1 and TSC2 (TSC1/2) stimulated mTORC2-dependent actin cytoskeleton organization and Paxillin phosphorylation. Furthermore, TSC1/2 siRNA increased mTORC2-dependent Ser473 phosphorylation of plasma membrane bound, myristoylated Akt/PKB. This suggests that loss of Akt/PKB Ser473 phosphorylation in TSC mutant cells, as reported previously, is due to inhibition of Akt/PKB localization rather than inhibition of mTORC2 activity. Amino acids and overexpression of Rheb failed to stimulate mTORC2 signaling. Thus, TSC1-TSC2 also inhibits mTORC2, but possibly independently of Rheb. Our results suggest that mTORC2 hyperactivation may contribute to the pathophysiology of diseases such as cancer and Tuberous Sclerosis Complex. i Acknowledgement During my PhD studies in the Biozentrum I received a lot of support from many people around me who I mention here to express my gratefulness. -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN.