The Simplified REHOS Cycle (Patent Pending)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcritical Pressure Organic Rankine Cycle (ORC) Analysis Based on the Integrated-Average Temperature Difference in Evaporators
Applied Thermal Engineering 88 (2015) 2e13 Contents lists available at ScienceDirect Applied Thermal Engineering journal homepage: www.elsevier.com/locate/apthermeng Research paper Transcritical pressure Organic Rankine Cycle (ORC) analysis based on the integrated-average temperature difference in evaporators * Chao Yu, Jinliang Xu , Yasong Sun The Beijing Key Laboratory of Multiphase Flow and Heat Transfer for Low Grade Energy Utilizations, North China Electric Power University, Beijing 102206, PR China article info abstract Article history: Integrated-average temperature difference (DTave) was proposed to connect with exergy destruction (Ieva) Received 24 June 2014 in heat exchangers. Theoretical expressions were developed for DTave and Ieva. Based on transcritical Received in revised form pressure ORCs, evaporators were theoretically studied regarding DTave. An exact linear relationship be- 12 October 2014 tween DT and I was identified. The increased specific heats versus temperatures for organic fluid Accepted 11 November 2014 ave eva protruded its TeQ curve to decrease DT . Meanwhile, the decreased specific heats concaved its TeQ Available online 20 November 2014 ave curve to raise DTave. Organic fluid in the evaporator undergoes a protruded TeQ curve and a concaved T eQ curve, interfaced at the pseudo-critical temperature point. Elongating the specific heat increment Keywords: fi Organic Rankine Cycle section and shortening the speci c heat decrease section improved the cycle performance. Thus, the fi Integrated-average temperature difference system thermal and exergy ef ciencies were increased by increasing critical temperatures for 25 organic Exergy destruction fluids. Wet fluids had larger thermal and exergy efficiencies than dry fluids, due to the fact that wet fluids Thermal match shortened the superheated vapor flow section in condensers. -

Rankine Cycle Definitions
Rankine Cycle Reading Problems 11.1 ! 11.7 11.29, 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74 Definitions • working fluid is alternately vaporized and condensed as it recirculates in a closed cycle • the standard vapour cycle that excludes internal irreversibilities is called the Ideal Rankine Cycle Analyze the Process Device 1st Law Balance Boiler h2 + qH = h3 ) qH = h3 − h2 (in) Turbine h3 = h4 + wT ) wT = h3 − h4 (out) Condenser h4 = h1 + qL ) qL = h4 − h1 (out) Pump h1 + wP = h2 ) wP = h2 − h1 (in) 1 The Rankine efficiency is net work output ηR = heat supplied to the boiler (h − h ) + (h − h ) = 3 4 1 2 (h3 − h2) Effects of Boiler and Condenser Pressure We know the efficiency is proportional to T η / 1 − L TH The question is ! how do we increase efficiency ) TL # and/or TH ". 1. INCREASED BOILER PRESSURE: • an increase in boiler pressure results in a higher TH for the same TL, therefore η ". • but 40 has a lower quality than 4 – wetter steam at the turbine exhaust – results in cavitation of the turbine blades – η # plus " maintenance • quality should be > 80 − 90% at the turbine exhaust 2 2. LOWER TL: • we are generally limited by the T ER (lake, river, etc.) eg. lake @ 15 ◦C + ∆T = 10 ◦C = 25 ◦C | {z } resistance to HT ) Psat = 3:169 kP a. • this is why we have a condenser – the pressure at the exit of the turbine can be less than atmospheric pressure 3. INCREASED TH BY ADDING SUPERHEAT: • the average temperature at which heat is supplied in the boiler can be increased by superheating the steam – dry saturated steam from the boiler is passed through a second bank of smaller bore tubes within the boiler until the steam reaches the required temperature – The value of T H , the mean temperature at which heat is added, increases, while TL remains constant. -

Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste
Sustainability 2014, 6, 5714-5729; doi:10.3390/su6095714 OPEN ACCESS sustainability ISSN 2071-1050 www.mdpi.com/journal/sustainability Article Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste Franco Cotana 1,†, Antonio Messineo 2,†, Alessandro Petrozzi 1,†,*, Valentina Coccia 1, Gianluca Cavalaglio 1 and Andrea Aquino 1 1 CRB, Centro di Ricerca sulle Biomasse, Via Duranti sn, 06125 Perugia, Italy; E-Mails: [email protected] (F.C.); [email protected] (V.C.); [email protected] (G.C.); [email protected] (A.A.) 2 Università degli Studi di Enna “Kore” Cittadella Universitaria, 94100 Enna, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-075-585-3806; Fax: +39-075-515-3321. Received: 25 June 2014; in revised form: 5 August 2014 / Accepted: 12 August 2014 / Published: 28 August 2014 Abstract: The Biomass Research Centre, section of CIRIAF, has recently developed a biomass boiler (300 kW thermal powered), fed by the poultry manure collected in a nearby livestock. All the thermal requirements of the livestock will be covered by the heat produced by gas combustion in the gasifier boiler. Within the activities carried out by the research project ENERPOLL (Energy Valorization of Poultry Manure in a Thermal Power Plant), funded by the Italian Ministry of Agriculture and Forestry, this paper aims at studying an upgrade version of the existing thermal plant, investigating and analyzing the possible applications for electricity production recovering the exceeding thermal energy. A comparison of Organic Rankine Cycle turbines and Stirling engines, to produce electricity from gasified poultry waste, is proposed, evaluating technical and economic parameters, considering actual incentives on renewable produced electricity. -

Rankine Cycle
MECH341: Thermodynamics of Engineering System Week 13 Chapter 10 Vapor & Combined Power Cycles The Carnot vapor cycle The Carnot cycle is the most efficient cycle operating between two specified temperature limits but it is not a suitable model for power cycles. Because: • Process 1-2 Limiting the heat transfer processes to two-phase systems severely limits the maximum temperature that can be used in the cycle (374°C for water) • Process 2-3 The turbine cannot handle steam with a high moisture content because of the impingement of liquid droplets on the turbine blades causing erosion and wear. • Process 4-1 It is not practical to design a compressor that handles two phases. The cycle in (b) is not suitable since it requires isentropic compression to extremely high pressures and isothermal heat transfer at variable pressures. 1-2 isothermal heat addition in a boiler 2-3 isentropic expansion in a turbine 3-4 isothermal heat rejection in a condenser 4-1 isentropic compression in a compressor T-s diagram of two Carnot vapor cycles. 1 Rankine cycle: The ideal cycle for vapor power cycles •Many of the impracticalities associated with the Carnot cycle can be eliminated by superheating the steam in the boiler and condensing it completely in the condenser. •The cycle that results is the Rankine cycle, which is the ideal cycle for vapor power plants. The ideal Rankine cycle does not involve any internal irreversibilities. Notes: • Only slight change in water temp through pump • The steam generator consists of boiler (two-phase heat transfer) and superheater • Turbine outlet is high-quality steam • Condenser is cooled by water (eg. -
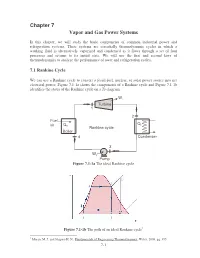
Chapter 7 Vapor and Gas Power Systems
Chapter 7 Vapor and Gas Power Systems In this chapter, we will study the basic components of common industrial power and refrigeration systems. These systems are essentially thermodyanmic cycles in which a working fluid is alternatively vaporized and condensed as it flows through a set of four processes and returns to its initial state. We will use the first and second laws of thermodynamics to analyze the performance of ower and refrigeration cycles. 7.1 Rankine Cycle We can use a Rankine cycle to convert a fossil-fuel, nuclear, or solar power source into net electrical power. Figure 7.1-1a shows the components of a Rankine cycle and Figure 7.1-1b identifies the states of the Rankine cycle on a Ts diagram. Wt Turbine 1 2 Fuel QC air QH Rankine cycle Boiler 4 Condenser 3 Wp Pump Figure 7.1-1a The ideal Rankine cycle Figure 7.1-1b The path of an ideal Rankine cycle1 1 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 395 7-1 In the ideal Rankine cycle, the working fluid undergoes four reversible processes: Process 1-2: Isentropic expansion of the working fluid from the turbine from saturated vapor at state 1 or superheated vapor at state 1’ to the condenser pressure. Process 2-3: Heat transfer from the working fluid as it flows at constant pressure through the condenser with saturated liquid at state 3. Process 3-4: Isentropic compression in the pump to state 4 in the compressed liquid region. Process 4-1: Heat transfer to the working fluid as it flows at constant pressure through the boiler to complete the cycle. -

Comparison Between Existing Rankine Cycle Refrigeration Systems and Hygroscopic Cycle Technology (HCT) †
Comparison between existing Rankine Cycle refrigeration systems and Hygroscopic Cycle Technology (HCT) † Francisco Javier Rubio Serrano 1, Fernando Soto Pérez 2 *, Antonio J. Gutiérrez Trashorras 3, Guillermo Ausin Abad 4 1IMASA, Ingeniería y Proyectos, S.A., Carpinteros 12, 28670 Villaviciosa de Odón, Madrid, Spain, [email protected] 2IMASA, Ingeniería y Proyectos, S.A., Palacio Valdés 1, 33002, Oviedo, Asturias, Spain, [email protected] 3 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] 4 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] * Corresponding Author e-mail: [email protected] ; Tel.: +34-696-467-104 † Presented at IRCSEEME, Universidad de Oviedo, Mieres del Camino, Asturias, España, 2018. Abstract: The objective of this paper is to review the different cooling systems that can be used in a Rankine cycle, especially the new technology called HCT (Hygroscopic Cycle Technology), that is based on the physical and chemical principles of absorption machines to increase the Rankine cycle net electrical efficiency and improve the cooling conditions. This technology allows an efficient and economical condensation of exhaust steam at the outlet of the steam turbine and significant decreases the water consumption. Advantages and high potential of HCT for power plants are analysed, comparing it with the current refrigeration systems. Also performances, investment and operation costs for each of the systems, are studied. Keywords: Refrigeration, Evaporative, Condenser, Hygroscopic, Absorber, Performance, Water, Dry-cooling, Rankine, Power. 1 1. Introduction Development of industries and increasing of population have produced a huge Energy demand. -

Organic Rankine Cycle Integration and Optimization for High Efficiency CHP Genset Systems
Organic Rankine Cycle Integration and Optimization for High Efficiency CHP Genset Systems Combined heat and power (CHP) systems Current Organic Rankine Cycle (ORC) systems operate at relatively low temperatures provide both electricity and heat for their and are used as a bottoming cycle waste heat recovery solution (top figure). The ORC host facilities. CHP systems have mostly system being developed can operate at higher temperatures and can thus be the saturated the large industrial facility primary recipient of the heat from a reciprocating engine (bottom figure). market, where economies of scale and the presence of needed technical staff make Photo credit ElectraTherm. the deployment of large systems greater than 20 megawatt (MW) electrical capacity a reciprocating engine to achieve total manufacturing sector analysis conducted cost effective and practical. There remains, CHP system efficiencies of 85% or more for the U.S. Department of Energy, however, substantial room for growth of at both its rated electrical capacity and at widespread deployment of flexible CHP smaller CHP systems suited for small and 50% capacity. To achieve this goal, the systems that are able to provide grid mid-size manufacturing facilities. system will be capable of operating in services could result in annual financial higher temperatures and pressures than benefits of approximately $1.4 billion In addition to manufacturing facility typical ORC systems. in the state of California alone. These energy benefits, the needs of the modern savings consist of lower industrial site electric grid are other potential drivers Benefits for Our Industry and energy costs, reduced grid operating costs, for further deployment of CHP systems. -

Review of Experimental Research on Supercritical and Transcritical Thermodynamic Cycles Designed for Heat Recovery Application
CORE Metadata, citation and similar papers at core.ac.uk Provided by Ghent University Academic Bibliography applied sciences Review Review of Experimental Research on Supercritical and Transcritical Thermodynamic Cycles Designed for Heat Recovery Application Steven Lecompte 1,2,* , Erika Ntavou 3, Bertrand Tchanche 4, George Kosmadakis 5 , Aditya Pillai 1,2 , Dimitris Manolakos 6 and Michel De Paepe 1,2 1 Department of Flow, Heat and Combustion Mechanics, Ghent University–UGent, Sint-Pietersnieuwstraat 41, 9000 Gent, Belgium; [email protected] (A.P.); [email protected] (M.D.P.) 2 Flanders Make, the Strategic Research Centre for the Manufacturing Industry, 3920 Lommel, Belgium 3 Department of Natural Resources and Agricultural Engineering, Agricultural University of Athens, Iera Odos 75, 11855 Athens, Greece; [email protected] 4 Department of Physics, Université Alioune Diop de Bambey, P.O. Box 30 Bambey, Senegal; [email protected] 5 Laboratory of Thermal Hydraulics and Multiphase Flow, INRASTES, National Center for Scientific Research “Demokritos”, Patriarchou Grigoriou and Neapoleos 27, 15341 Agia Paraskevi, Greece; [email protected] 6 Department of Natural Resources and Agricultural Engineering, Agricultural University of Athens, Iera Odos 75, 11855 Athens, Greece; [email protected] * Correspondence: [email protected]; Tel.: +32-470045524 Received: 21 May 2019; Accepted: 17 June 2019; Published: 25 June 2019 Abstract: Supercritical operation is considered a main technique to achieve higher cycle efficiency in various thermodynamic systems. The present paper is a review of experimental investigations on supercritical operation considering both heat-to-upgraded heat and heat-to-power systems. Experimental works are reported and subsequently analyzed. -

Parabolic Dish Solar Thermal Power Annual Program Review Proceedings
5105 -83 DOE/JPL 1060-46 Solar Thermal Power Systems Project D~stributionCategory UC-62 Parabolic Dlsh Systems Devebpment Parabolic Dish Solar Thermal Power Annual Program Review Proceedings May 1,1981 Prepared for U.S. Department of Energy Through an agreement wlih Natlonal Aeronautics and Space Adm~nistration by Jet Propuls~onLaboratory Cal~forn~aInsh, ~teof Technology Pasadena. Cahfornia (JPL F'UBLICATION 81-44) 5105 -83 DOE/JPL U60-46 Solar Thermal Power Systems Project Distribution Category UC-62 Parabolic Dish Systems Development .I :: # a -. i- Parabolic Dish 3.*. ' i Solar Thermal Power . *_ ~ a h Annual Program Review Proceedings May 1,1981 Prepared for U S. Department of Energy Throuah an aareement wltk ~at106al~eroiautics and Space Admmistration by Jet Propulsion Laboratory Callforn~alnstltute of Technology Pasadena, Callfornla (JPI . PUBLICATION 81-44) Prepared by the Jet Propulsion khontory, California Institute of Technology, for the U.S Department of Energy through an agreement with the Natrond Aere nautics and Space Administration. The JPL Solar Thermal Power Systems Project is sponsored by the U.S. Depart- ment of Energy and fxms a part of :he Soh Thermal Rognm to develop low- cost solar thermal and electric power plantr Thb report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors. subcontractors, or theu employees. makes any warranty, cxpress or implied, or assumes any legal liability or respons~bilityfor the accuracy, completeness or usefulness of any in- formation, apparatus. -

Cascaded Transcritical/Supercritical CO2 Cycles and Organic Rankine Cycles to Recover Low-Temperature Waste Heat and LNG Cold En
World Academy of Science, Engineering and Technology International Journal of Energy and Power Engineering Vol:12, No:4, 2018 Cascaded Transcritical/Supercritical CO2 Cycles and Organic Rankine Cycles to Recover Low-Temperature Waste Heat and LNG Cold Energy Simultaneously Haoshui Yu, Donghoi Kim, Truls Gundersen point in the evaporator and poor performance for sensible heat Abstract—Low-temperature waste heat is abundant in the process sources. Transcritical and supercritical CO2 cycles may industries, and large amounts of Liquefied Natural Gas (LNG) cold perform better to convert low-temperature waste heat into energy are discarded without being recovered properly in LNG electricity because of better temperature glide matching terminals. Power generation is an effective way to utilize between waste heat and CO without pinch limitations. CO has low-temperature waste heat and LNG cold energy simultaneously. 2 2 no toxicity, no flammability, it is not explosive, easy to obtain, Organic Rankine Cycles (ORCs) and CO2 power cycles are promising technologies to convert low-temperature waste heat and LNG cold and when used in a cycle it has no negative effect on the energy into electricity. If waste heat and LNG cold energy are utilized environment [3]. However, due to its low critical temperature, simultaneously in one system, the performance may outperform the condensation of CO2 is a vital problem in practice. CO2 separate systems utilizing low-temperature waste heat and LNG cold power cycles have considerable potential for low-temperature energy, respectively. Low-temperature waste heat acts as the heat heat recovery if the proper heat sink is available. Nevertheless, source and LNG regasification acts as the heat sink in the combined system. -

Vapor Power Cycles Ideal Rankine Cycle
Vapor Power Cycles We know that the Carnot cycle is most efficient cycle operating between two specified temperature limits. However; the Carnot cycle is not a suitable model for steam power cycle since: The turbine has to handle steam with low quality which will cause erosion and wear in turbine blades. It is impractical to design a compressor that handles two phase. It is difficult to control the condensation process that precisely as to end up with the desired at point 4. T 1 2 1 2 4 3 4 3 s s Fig. 1: T-s diagram for two Carnot vapor cycle. Other issues include: isentropic compression to extremely high pressure and isothermal heat transfer at variable pressures. Thus, the Carnot cycle cannot be approximated in actual devices and is not a realistic model for vapor power cycles. Ideal Rankine Cycle The Rankine cycle is the ideal cycle for vapor power plants; it includes the following four reversible processes: 1-2: Isentropic compression Water enters the pump as state 1 as saturated liquid and is compressed isentropically to the operating pressure of the boiler. 2-3: Const P heat addition Saturated water enters the boiler and leaves it as superheated vapor at state 3 3-4: Isentropic expansion Superheated vapor expands isentropically in turbine and produces work. 4-1: Const P heat rejection High quality steam is condensed in the condenser M. Bahrami ENSC 461 (S 11) Vapor Power Cycles 1 Boiler T 3 Turbine Wout Q in Qin 2 Wout Pump Win 4 Win 1 Qout Condenser Qout s Fig. -

Rankine Cycle Definitions Effects of Boiler and Condenser Pressure
Rankine Cycle Reading Problems 10-2 ! 10-7 10-19, 10-38, 10-41, 10-52, 10-53, 10-64 Definitions • the standard vapour cycle that excludes internal irreversibilities is called the Ideal Rankine Cycle Effects of Boiler and Condenser Pressure 1. INCREASED BOILER PRESSURE: • an increase in boiler pressure results in a higher TH for the same TL, therefore η ". • but 40 has a lower quality than 4 – wetter steam at the turbine exhaust – results in cavitation of the turbine blades – η # plus " maintenance • quality should be > 80 − 90% at the turbine exhaust 1 2. LOWER TL: • we are generally limited by the T ER (lake, river, etc.) eg. lake @ 15 ◦C + ∆T = 10 ◦C = 25 ◦C | {z } resistance to HT ) Psat = 3:2 kP a. • this is why we have a condenser – the pressure at the exit of the turbine can be less than atmospheric pressure – the closed loop of the condenser allows us to use treated water on the cycle side – but if the pressure is less that atmospheric pressure, air can leak into the condenser, preventing condensation 3. INCREASED TH BY ADDING SUPERHEAT: • the average temperature at which heat is supplied in the boiler can be increased by superheating the steam 2 – dry saturated steam from the boiler is passed through a second bank of smaller bore tubes within the boiler until the steam reaches the required temperature The advantage is W " η = net overall " QH " The value of T H , the mean temperature at which heat is added, increases, while TL remains constant. Therefore the efficiency increases.