Transcritical Pressure Organic Rankine Cycle (ORC) Analysis Based on the Integrated-Average Temperature Difference in Evaporators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamic Optimization of an Organic Rankine Cycle for Power Generation from a Low Temperature Geothermal Heat Source
Thermodynamic Optimization of an Organic Rankine Cycle for Power Generation from a Low Temperature Geothermal Heat Source Inés Encabo Cáceres Roberto Agromayor Lars O. Nord Department of Energy and Process Engineering Norwegian University of Science and Technology (NTNU) Kolbjørn Hejes v.1B, NO-7491 Trondheim, Norway [email protected] Abstract fueled by natural gas or other fossil fuels (Macchi and Astolfi, 2017). However, during the last years, the The increasing concern on environment problems has increasing concern of the greenhouse effect and climate led to the development of renewable energy sources, change has led to an increase of renewable energy, such being the geothermal energy one of the most promising as wind and solar power. In addition to these listed ones in terms of power generation. Due to the low heat renewable energies, there is an energy source that shows source temperatures this energy provides, the use of a promising future due to the advantages it provides Organic Rankine Cycles is necessary to guarantee a when compared to other renewable energies. This good performance of the system. In this paper, the developing energy is geothermal energy, and its optimization of an Organic Rankine Cycle has been advantages are related to its availability (Macchi and carried out to determine the most suitable working fluid. Astolfi, 2017): it does not depend on the ambient Different cycle layouts and configurations for 39 conditions, it is stable, and it offers the possibility of different working fluids were simulated by means of a renewable energy base load operation. One of the Gradient Based Optimization Algorithm implemented challenges of geothermal energy is that it does not in MATLAB and linked to REFPROP property library. -

Sustainable Energy Conversion Through the Use of Organic Rankine Cycles for Waste Heat Recovery and Solar Applications
ENERGY SYSTEMS RESEARCH UNIT AEROSPACE AND MECHANICAL ENGINEERING DEPARTMENT UNIVERSITY OF LIÈGE Sustainable Energy Conversion Through the Use of Organic Rankine Cycles for Waste Heat Recovery and Solar Applications. in Partial Fulfillment of the Requirements for the Degree of Doctor of Applied Sciences Presented to the Faculty of Applied Science of the University of Liège (Belgium) by Sylvain Quoilin Liège, October 2011 Introductory remarks Version 1.1, published in October 2011 © 2011 Sylvain Quoilin 〈►[email protected]〉 Licence. This work is licensed under the Creative Commons Attribution - No Derivative Works 2.5 License. To view a copy of this license, visit ►http://creativecommons.org/licenses/by-nd/2.5/ or send a letter to Creative Commons, 543 Howard Street, 5th Floor, San Francisco, California, 94105, USA. Contact the author to request other uses if necessary. Trademarks and service marks. All trademarks, service marks, logos and company names mentioned in this work are property of their respective owner. They are protected under trademark law and unfair competition law. The importance of the glossary. It is strongly recommended to read the glossary in full before starting with the first chapter. Hints for screen use. This work is optimized for both screen and paper use. It is recommended to use the digital version where applicable. It is a file in Portable Document Format (PDF) with hyperlinks for convenient navigation. All hyperlinks are marked with link flags (►). Hyperlinks in diagrams might be marked with colored borders instead. Navigation aid for bibliographic references. Bibliographic references to works which are publicly available as PDF files mention the logical page number and an offset (if non-zero) to calculate the physical page number. -

Rankine Cycle Definitions
Rankine Cycle Reading Problems 11.1 ! 11.7 11.29, 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74 Definitions • working fluid is alternately vaporized and condensed as it recirculates in a closed cycle • the standard vapour cycle that excludes internal irreversibilities is called the Ideal Rankine Cycle Analyze the Process Device 1st Law Balance Boiler h2 + qH = h3 ) qH = h3 − h2 (in) Turbine h3 = h4 + wT ) wT = h3 − h4 (out) Condenser h4 = h1 + qL ) qL = h4 − h1 (out) Pump h1 + wP = h2 ) wP = h2 − h1 (in) 1 The Rankine efficiency is net work output ηR = heat supplied to the boiler (h − h ) + (h − h ) = 3 4 1 2 (h3 − h2) Effects of Boiler and Condenser Pressure We know the efficiency is proportional to T η / 1 − L TH The question is ! how do we increase efficiency ) TL # and/or TH ". 1. INCREASED BOILER PRESSURE: • an increase in boiler pressure results in a higher TH for the same TL, therefore η ". • but 40 has a lower quality than 4 – wetter steam at the turbine exhaust – results in cavitation of the turbine blades – η # plus " maintenance • quality should be > 80 − 90% at the turbine exhaust 2 2. LOWER TL: • we are generally limited by the T ER (lake, river, etc.) eg. lake @ 15 ◦C + ∆T = 10 ◦C = 25 ◦C | {z } resistance to HT ) Psat = 3:169 kP a. • this is why we have a condenser – the pressure at the exit of the turbine can be less than atmospheric pressure 3. INCREASED TH BY ADDING SUPERHEAT: • the average temperature at which heat is supplied in the boiler can be increased by superheating the steam – dry saturated steam from the boiler is passed through a second bank of smaller bore tubes within the boiler until the steam reaches the required temperature – The value of T H , the mean temperature at which heat is added, increases, while TL remains constant. -

Fluid Selection of Transcritical Rankine Cycle for Engine Waste Heat Recovery Based on Temperature Match Method
energies Article Fluid Selection of Transcritical Rankine Cycle for Engine Waste Heat Recovery Based on Temperature Match Method Zhijian Wang 1, Hua Tian 2, Lingfeng Shi 3, Gequn Shu 2, Xianghua Kong 1 and Ligeng Li 2,* 1 State Key Laboratory of Engine Reliability, Weichai Power Co., Ltd., Weifang 261001, China; [email protected] (Z.W.); [email protected] (X.K.) 2 State Key Laboratory of Engines, Tianjin University, Tianjin 300072, China; [email protected] (H.T.); [email protected] (G.S.) 3 Department of Thermal Science and Energy Engineering, University of Science and Technology of China, Hefei 230027, China; [email protected] * Correspondence: [email protected] Received: 6 March 2020; Accepted: 8 April 2020; Published: 10 April 2020 Abstract: Engines waste a major part of their fuel energy in the jacket water and exhaust gas. Transcritical Rankine cycles are a promising technology to recover the waste heat efficiently. The working fluid selection seems to be a key factor that determines the system performances. However, most of the studies are mainly devoted to compare their thermodynamic performances of various fluids and to decide what kind of properties the best-working fluid shows. In this work, an active working fluid selection instruction is proposed to deal with the temperature match between the bottoming system and cold source. The characters of ideal working fluids are summarized firstly when the temperature match method of a pinch analysis is combined. Various selected fluids are compared in thermodynamic and economic performances to verify the fluid selection instruction. It is found that when the ratio of the average specific heat in the heat transfer zone of exhaust gas to the average specific heat in the heat transfer zone of jacket water becomes higher, the irreversibility loss between the working fluid and cold source is improved. -

Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste
Sustainability 2014, 6, 5714-5729; doi:10.3390/su6095714 OPEN ACCESS sustainability ISSN 2071-1050 www.mdpi.com/journal/sustainability Article Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste Franco Cotana 1,†, Antonio Messineo 2,†, Alessandro Petrozzi 1,†,*, Valentina Coccia 1, Gianluca Cavalaglio 1 and Andrea Aquino 1 1 CRB, Centro di Ricerca sulle Biomasse, Via Duranti sn, 06125 Perugia, Italy; E-Mails: [email protected] (F.C.); [email protected] (V.C.); [email protected] (G.C.); [email protected] (A.A.) 2 Università degli Studi di Enna “Kore” Cittadella Universitaria, 94100 Enna, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-075-585-3806; Fax: +39-075-515-3321. Received: 25 June 2014; in revised form: 5 August 2014 / Accepted: 12 August 2014 / Published: 28 August 2014 Abstract: The Biomass Research Centre, section of CIRIAF, has recently developed a biomass boiler (300 kW thermal powered), fed by the poultry manure collected in a nearby livestock. All the thermal requirements of the livestock will be covered by the heat produced by gas combustion in the gasifier boiler. Within the activities carried out by the research project ENERPOLL (Energy Valorization of Poultry Manure in a Thermal Power Plant), funded by the Italian Ministry of Agriculture and Forestry, this paper aims at studying an upgrade version of the existing thermal plant, investigating and analyzing the possible applications for electricity production recovering the exceeding thermal energy. A comparison of Organic Rankine Cycle turbines and Stirling engines, to produce electricity from gasified poultry waste, is proposed, evaluating technical and economic parameters, considering actual incentives on renewable produced electricity. -

APPLICATION to ORGANIC RANKINE CYCLES TURBINES Pietro Marco Congedo
ANALYSIS AND OPTIMIZATION OF DENSE GAS FLOWS: APPLICATION TO ORGANIC RANKINE CYCLES TURBINES Pietro Marco Congedo To cite this version: Pietro Marco Congedo. ANALYSIS AND OPTIMIZATION OF DENSE GAS FLOWS: APPLICA- TION TO ORGANIC RANKINE CYCLES TURBINES. Modeling and Simulation. Università degli studi di Lecce, 2007. English. tel-00349762 HAL Id: tel-00349762 https://tel.archives-ouvertes.fr/tel-00349762 Submitted on 4 Jan 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITA’ DEL SALENTO Dipartimento di Ingegneria dell’Innovazione CREA – Centro Ricerche Energie ed Ambiente Ph.D. Thesis in “Sistemi Energetici ed Ambiente” XIX Ciclo ANALYSIS AND OPTIMIZATION OF DENSE GAS FLOWS: APPLICATION TO ORGANIC RANKINE CYCLES TURBINES Coordinatore del Ph.D. Ch.mo Prof. Ing. Domenico Laforgia Tutor Ch.ma Prof.ssa Ing. Paola Cinnella Studente Ing. Pietro Marco Congedo Anno Accademico 2006/2007 A tutti coloro che credono in me. Io continuerò sempre a credere in loro. 2 ANALYSIS AND OPTIMIZATION OF DENSE GAS FLOWS: APPLICATION TO ORGANIC RANKINE CYCLES TURBINES by Pietro Marco Congedo (ABSTRACT) This thesis presents an accurate study about the fluid-dynamics of dense gases and their potential application as working fluids in Organic Rankine Cycles (ORCs). -

Comparison of the Organic Flash Cycle (OFC) to Other Advanced Vapor Cycles for Intermediate and High Temperature Waste Heat Reclamation and Solar Thermal Energy
Energy 42 (2012) 213e223 Contents lists available at SciVerse ScienceDirect Energy journal homepage: www.elsevier.com/locate/energy Comparison of the Organic Flash Cycle (OFC) to other advanced vapor cycles for intermediate and high temperature waste heat reclamation and solar thermal energy Tony Ho*, Samuel S. Mao, Ralph Greif Department of Mechanical Engineering, University of California-Berkeley, Etcheverry Hall, Berkeley, CA 94720, USA article info abstract Article history: The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially improve the Received 27 August 2011 efficiency with which high and intermediate temperature finite thermal sources are utilized. The OFC’s Received in revised form aim is to improve temperature matching and reduce exergy losses during heat addition. A theoretical 22 February 2012 investigation is conducted using high accuracy equations of state such as BACKONE, SpaneWagner, and Accepted 24 March 2012 REFPROP in a detailed thermodynamic and exergetic analysis. The study examines 10 different aromatic Available online 22 April 2012 hydrocarbons and siloxanes as potential working fluids. Comparisons are drawn between the OFC and an optimized basic Organic Rankine Cycle (ORC), a zeotropic Rankine cycle using a binary ammonia-water Keywords: Organic Rankine Cycle (ORC) mixture, and a transcritical CO2 cycle. Results showed aromatic hydrocarbons to be the better suited fl Solar thermal working uid for the ORC and OFC due to higher power output and less complex turbine designs. Results Waste heat also showed that the single flash OFC achieves comparable utilization efficiencies to the optimized basic Vapor cycle ORC. Although the OFC improved heat addition exergetic efficiency, this advantage was negated by Exergy analysis irreversibilities introduced during flash evaporation. -

Rankine Cycle
MECH341: Thermodynamics of Engineering System Week 13 Chapter 10 Vapor & Combined Power Cycles The Carnot vapor cycle The Carnot cycle is the most efficient cycle operating between two specified temperature limits but it is not a suitable model for power cycles. Because: • Process 1-2 Limiting the heat transfer processes to two-phase systems severely limits the maximum temperature that can be used in the cycle (374°C for water) • Process 2-3 The turbine cannot handle steam with a high moisture content because of the impingement of liquid droplets on the turbine blades causing erosion and wear. • Process 4-1 It is not practical to design a compressor that handles two phases. The cycle in (b) is not suitable since it requires isentropic compression to extremely high pressures and isothermal heat transfer at variable pressures. 1-2 isothermal heat addition in a boiler 2-3 isentropic expansion in a turbine 3-4 isothermal heat rejection in a condenser 4-1 isentropic compression in a compressor T-s diagram of two Carnot vapor cycles. 1 Rankine cycle: The ideal cycle for vapor power cycles •Many of the impracticalities associated with the Carnot cycle can be eliminated by superheating the steam in the boiler and condensing it completely in the condenser. •The cycle that results is the Rankine cycle, which is the ideal cycle for vapor power plants. The ideal Rankine cycle does not involve any internal irreversibilities. Notes: • Only slight change in water temp through pump • The steam generator consists of boiler (two-phase heat transfer) and superheater • Turbine outlet is high-quality steam • Condenser is cooled by water (eg. -
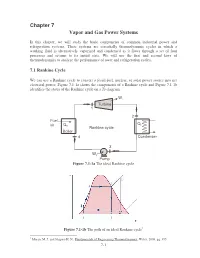
Chapter 7 Vapor and Gas Power Systems
Chapter 7 Vapor and Gas Power Systems In this chapter, we will study the basic components of common industrial power and refrigeration systems. These systems are essentially thermodyanmic cycles in which a working fluid is alternatively vaporized and condensed as it flows through a set of four processes and returns to its initial state. We will use the first and second laws of thermodynamics to analyze the performance of ower and refrigeration cycles. 7.1 Rankine Cycle We can use a Rankine cycle to convert a fossil-fuel, nuclear, or solar power source into net electrical power. Figure 7.1-1a shows the components of a Rankine cycle and Figure 7.1-1b identifies the states of the Rankine cycle on a Ts diagram. Wt Turbine 1 2 Fuel QC air QH Rankine cycle Boiler 4 Condenser 3 Wp Pump Figure 7.1-1a The ideal Rankine cycle Figure 7.1-1b The path of an ideal Rankine cycle1 1 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 395 7-1 In the ideal Rankine cycle, the working fluid undergoes four reversible processes: Process 1-2: Isentropic expansion of the working fluid from the turbine from saturated vapor at state 1 or superheated vapor at state 1’ to the condenser pressure. Process 2-3: Heat transfer from the working fluid as it flows at constant pressure through the condenser with saturated liquid at state 3. Process 3-4: Isentropic compression in the pump to state 4 in the compressed liquid region. Process 4-1: Heat transfer to the working fluid as it flows at constant pressure through the boiler to complete the cycle. -

Comparison Between Existing Rankine Cycle Refrigeration Systems and Hygroscopic Cycle Technology (HCT) †
Comparison between existing Rankine Cycle refrigeration systems and Hygroscopic Cycle Technology (HCT) † Francisco Javier Rubio Serrano 1, Fernando Soto Pérez 2 *, Antonio J. Gutiérrez Trashorras 3, Guillermo Ausin Abad 4 1IMASA, Ingeniería y Proyectos, S.A., Carpinteros 12, 28670 Villaviciosa de Odón, Madrid, Spain, [email protected] 2IMASA, Ingeniería y Proyectos, S.A., Palacio Valdés 1, 33002, Oviedo, Asturias, Spain, [email protected] 3 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] 4 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] * Corresponding Author e-mail: [email protected] ; Tel.: +34-696-467-104 † Presented at IRCSEEME, Universidad de Oviedo, Mieres del Camino, Asturias, España, 2018. Abstract: The objective of this paper is to review the different cooling systems that can be used in a Rankine cycle, especially the new technology called HCT (Hygroscopic Cycle Technology), that is based on the physical and chemical principles of absorption machines to increase the Rankine cycle net electrical efficiency and improve the cooling conditions. This technology allows an efficient and economical condensation of exhaust steam at the outlet of the steam turbine and significant decreases the water consumption. Advantages and high potential of HCT for power plants are analysed, comparing it with the current refrigeration systems. Also performances, investment and operation costs for each of the systems, are studied. Keywords: Refrigeration, Evaporative, Condenser, Hygroscopic, Absorber, Performance, Water, Dry-cooling, Rankine, Power. 1 1. Introduction Development of industries and increasing of population have produced a huge Energy demand. -

Modifications and Optimization of the Organic Rankine Cycle
Modifications and Optimization of the Organic Rankine Cycle Improving Recovery of Waste Heat in Industrial Processes This project developed and demonstrated a direct evapora- tor for an Organic Rankine Cycle (ORC) system that utilizes waste heat from a reciprocating engine or gas turbine. Introduction Waste heat from turbines and engines used in industrial ap- plications along with waste heat from industrial processes are exceptionally abundant sources of energy. If even a fraction of this waste heat could be economically converted to useful electricity, it would have a tangible and very positive impact on the economic health, energy consumption, and carbon emis- sions in the U.S. manufacturing sector. The current waste heat recovery technologies, including ORC systems and thermoelec- The direct evaporator and supporting equipment installed at the trics, are technically feasible but in many cases economically GE test facility. Photo courtesy of Idaho National Laboratory. unattractive. This limits their current use to a small number of niche applications. economic benefit of this research effort is a reduction in the costs ORC technologies operate by transferring heat from the source of ORC technology, which will provide greater returns on invest- through an evaporator to boil a fluid and create vapor that is ment than previous ORC systems. expanded across a turbine or other work extraction device. This creates shaft power that can be easily turned into electrical power through a generator. Current limitations in ORC technolo- Applications in Our Nation’s Industry gies have led to inefficient systems that offer only marginal This technology will be initially retrofitted for waste heat recov- economic benefits. -

Comparative Analysis of Small-Scale Integrated Solar ORC-Absorption Based Cogeneration Systems
energies Article Comparative Analysis of Small-Scale Integrated Solar ORC-Absorption Based Cogeneration Systems Xiaoqiang Hong * and Feng Shi School of Architecture and Civil Engineering, Xiamen University, Xiamen 361005, China; [email protected] * Correspondence: [email protected]; Tel.: +86-592-218-8958 Received: 16 January 2020; Accepted: 18 February 2020; Published: 20 February 2020 Abstract: This paper aims to present a comparative study into the cascade and series configurations of the organic Rankine cycle based small-scale solar combined cooling, heating and power system for civil application. The energy performance of the systems is studied by developing a thermodynamic model. The simulation model is validated using the literature results. Analyses of the research results indicated that the cascade system can achieve maximum value of the primary energy efficiency of 13.4% for cooling and power generation under solar collecting temperature of 115 ◦C in cooling mode. The cascade system has more cooling output and less electricity output in cooling mode compared with the series system. In heating mode, the single solar organic Rankine cycle (ORC) operation can achieve highest primary energy efficiency of 19.6% for heating and power generation under solar collecting temperature of 100 ◦C. Systems with R141b as ORC working fluid show better performance than those with R123 and R1233zd(E). Keywords: solar cooling; ORC; absorption chiller; CPC 1. Introduction Building sectors consumed a large amount of non-renewable energy resources. Regarded as one of the most feasible renewable solutions for the building application, solar thermal technology is proven to be the most mature technology among all currently available solar technologies, for meeting building’s electricity and hot water demand.