An Analysis of a Thermal Power Plant Working on a Rankine Cycle: a Theoretical Investigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcritical Pressure Organic Rankine Cycle (ORC) Analysis Based on the Integrated-Average Temperature Difference in Evaporators
Applied Thermal Engineering 88 (2015) 2e13 Contents lists available at ScienceDirect Applied Thermal Engineering journal homepage: www.elsevier.com/locate/apthermeng Research paper Transcritical pressure Organic Rankine Cycle (ORC) analysis based on the integrated-average temperature difference in evaporators * Chao Yu, Jinliang Xu , Yasong Sun The Beijing Key Laboratory of Multiphase Flow and Heat Transfer for Low Grade Energy Utilizations, North China Electric Power University, Beijing 102206, PR China article info abstract Article history: Integrated-average temperature difference (DTave) was proposed to connect with exergy destruction (Ieva) Received 24 June 2014 in heat exchangers. Theoretical expressions were developed for DTave and Ieva. Based on transcritical Received in revised form pressure ORCs, evaporators were theoretically studied regarding DTave. An exact linear relationship be- 12 October 2014 tween DT and I was identified. The increased specific heats versus temperatures for organic fluid Accepted 11 November 2014 ave eva protruded its TeQ curve to decrease DT . Meanwhile, the decreased specific heats concaved its TeQ Available online 20 November 2014 ave curve to raise DTave. Organic fluid in the evaporator undergoes a protruded TeQ curve and a concaved T eQ curve, interfaced at the pseudo-critical temperature point. Elongating the specific heat increment Keywords: fi Organic Rankine Cycle section and shortening the speci c heat decrease section improved the cycle performance. Thus, the fi Integrated-average temperature difference system thermal and exergy ef ciencies were increased by increasing critical temperatures for 25 organic Exergy destruction fluids. Wet fluids had larger thermal and exergy efficiencies than dry fluids, due to the fact that wet fluids Thermal match shortened the superheated vapor flow section in condensers. -

Physics 170 - Thermodynamic Lecture 40
Physics 170 - Thermodynamic Lecture 40 ! The second law of thermodynamic 1 The Second Law of Thermodynamics and Entropy There are several diferent forms of the second law of thermodynamics: ! 1. In a thermal cycle, heat energy cannot be completely transformed into mechanical work. ! 2. It is impossible to construct an operational perpetual-motion machine. ! 3. It’s impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body ! 4. Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. ! ! Heat Engines and Thermal Pumps A heat engine converts heat energy into work. According to the second law of thermodynamics, however, it cannot convert *all* of the heat energy supplied to it into work. Basic heat engine: hot reservoir, cold reservoir, and a machine to convert heat energy into work. Heat Engines and Thermal Pumps 4 Heat Engines and Thermal Pumps This is a simplified diagram of a heat engine, along with its thermal cycle. Heat Engines and Thermal Pumps An important quantity characterizing a heat engine is the net work it does when going through an entire cycle. Heat Engines and Thermal Pumps Heat Engines and Thermal Pumps Thermal efciency of a heat engine: ! ! ! ! ! ! From the first law, it follows: Heat Engines and Thermal Pumps Yet another restatement of the second law of thermodynamics: No cyclic heat engine can convert its heat input completely to work. Heat Engines and Thermal Pumps A thermal pump is the opposite of a heat engine: it transfers heat energy from a cold reservoir to a hot one. -

Physics 100 Lecture 7
2 Physics 100 Lecture 7 Heat Engines and the 2nd Law of Thermodynamics February 12, 2018 3 Thermal Convection Warm fluid is less dense and rises while cool fluid sinks Resulting circulation efficiently transports thermal energy 4 COLD Convection HOT Turbulent motion of glycerol in a container heated from below and cooled from above. The bright lines show regions of rapid temperature variation. The fluid contains many "plumes," especially near the walls. The plumes can be identified as mushroom-shaped objects with heat flowing through the "stalk" and spreading in the "cap." The hot plumes tend to rise with their caps on top; falling, cold plumes are cap-down. All this plume activity is carried along in an overall counterclockwise "wind" caused by convection. Note the thermometer coming down from the top of the cell. Figure adapted from J. Zhang, S. Childress, A. Libchaber, Phys. Fluids 9, 1034 (1997). See detailed discussion in Kadanoff, L. P., Physics Today 54, 34 (August 2001). 5 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B. onshore … offshore C. offshore … onshore D. offshore … offshore 6 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B.onshore … offshore C.offshore … onshore D.offshore … offshore 7 Thermal radiation Any object whose temperature is above zero Kelvin emits energy in the form of electromagnetic radiation Objects both absorb and emit EM radiation continuously, and this phenomenon helps determine the object’s equilibrium temperature 8 The electromagnetic spectrum 9 Thermal radiation We’ll examine this concept some more in chapter 6 10 Why does the Earth cool more quickly on clear nights than it does on cloudy nights? A. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

Rankine Cycle Definitions
Rankine Cycle Reading Problems 11.1 ! 11.7 11.29, 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74 Definitions • working fluid is alternately vaporized and condensed as it recirculates in a closed cycle • the standard vapour cycle that excludes internal irreversibilities is called the Ideal Rankine Cycle Analyze the Process Device 1st Law Balance Boiler h2 + qH = h3 ) qH = h3 − h2 (in) Turbine h3 = h4 + wT ) wT = h3 − h4 (out) Condenser h4 = h1 + qL ) qL = h4 − h1 (out) Pump h1 + wP = h2 ) wP = h2 − h1 (in) 1 The Rankine efficiency is net work output ηR = heat supplied to the boiler (h − h ) + (h − h ) = 3 4 1 2 (h3 − h2) Effects of Boiler and Condenser Pressure We know the efficiency is proportional to T η / 1 − L TH The question is ! how do we increase efficiency ) TL # and/or TH ". 1. INCREASED BOILER PRESSURE: • an increase in boiler pressure results in a higher TH for the same TL, therefore η ". • but 40 has a lower quality than 4 – wetter steam at the turbine exhaust – results in cavitation of the turbine blades – η # plus " maintenance • quality should be > 80 − 90% at the turbine exhaust 2 2. LOWER TL: • we are generally limited by the T ER (lake, river, etc.) eg. lake @ 15 ◦C + ∆T = 10 ◦C = 25 ◦C | {z } resistance to HT ) Psat = 3:169 kP a. • this is why we have a condenser – the pressure at the exit of the turbine can be less than atmospheric pressure 3. INCREASED TH BY ADDING SUPERHEAT: • the average temperature at which heat is supplied in the boiler can be increased by superheating the steam – dry saturated steam from the boiler is passed through a second bank of smaller bore tubes within the boiler until the steam reaches the required temperature – The value of T H , the mean temperature at which heat is added, increases, while TL remains constant. -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

Power Plant Steam Cycle Theory - R.A
THERMAL POWER PLANTS – Vol. I - Power Plant Steam Cycle Theory - R.A. Chaplin POWER PLANT STEAM CYCLE THEORY R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Steam Turbines, Carnot Cycle, Rankine Cycle, Superheating, Reheating, Feedwater Heating. Contents 1. Cycle Efficiencies 1.1. Introduction 1.2. Carnot Cycle 1.3. Simple Rankine Cycles 1.4. Complex Rankine Cycles 2. Turbine Expansion Lines 2.1. T-s and h-s Diagrams 2.2. Turbine Efficiency 2.3. Turbine Configuration 2.4. Part Load Operation Glossary Bibliography Biographical Sketch Summary The Carnot cycle is an ideal thermodynamic cycle based on the laws of thermodynamics. It indicates the maximum efficiency of a heat engine when operating between given temperatures of heat acceptance and heat rejection. The Rankine cycle is also an ideal cycle operating between two temperature limits but it is based on the principle of receiving heat by evaporation and rejecting heat by condensation. The working fluid is water-steam. In steam driven thermal power plants this basic cycle is modified by incorporating superheating and reheating to improve the performance of the turbine. UNESCO – EOLSS The Rankine cycle with its modifications suggests the best efficiency that can be obtained from this two phaseSAMPLE thermodynamic cycle wh enCHAPTERS operating under given temperature limits but its efficiency is less than that of the Carnot cycle since some heat is added at a lower temperature. The efficiency of the Rankine cycle can be improved by regenerative feedwater heating where some steam is taken from the turbine during the expansion process and used to preheat the feedwater before it is evaporated in the boiler. -

Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste
Sustainability 2014, 6, 5714-5729; doi:10.3390/su6095714 OPEN ACCESS sustainability ISSN 2071-1050 www.mdpi.com/journal/sustainability Article Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste Franco Cotana 1,†, Antonio Messineo 2,†, Alessandro Petrozzi 1,†,*, Valentina Coccia 1, Gianluca Cavalaglio 1 and Andrea Aquino 1 1 CRB, Centro di Ricerca sulle Biomasse, Via Duranti sn, 06125 Perugia, Italy; E-Mails: [email protected] (F.C.); [email protected] (V.C.); [email protected] (G.C.); [email protected] (A.A.) 2 Università degli Studi di Enna “Kore” Cittadella Universitaria, 94100 Enna, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-075-585-3806; Fax: +39-075-515-3321. Received: 25 June 2014; in revised form: 5 August 2014 / Accepted: 12 August 2014 / Published: 28 August 2014 Abstract: The Biomass Research Centre, section of CIRIAF, has recently developed a biomass boiler (300 kW thermal powered), fed by the poultry manure collected in a nearby livestock. All the thermal requirements of the livestock will be covered by the heat produced by gas combustion in the gasifier boiler. Within the activities carried out by the research project ENERPOLL (Energy Valorization of Poultry Manure in a Thermal Power Plant), funded by the Italian Ministry of Agriculture and Forestry, this paper aims at studying an upgrade version of the existing thermal plant, investigating and analyzing the possible applications for electricity production recovering the exceeding thermal energy. A comparison of Organic Rankine Cycle turbines and Stirling engines, to produce electricity from gasified poultry waste, is proposed, evaluating technical and economic parameters, considering actual incentives on renewable produced electricity. -

Rankine Cycle
MECH341: Thermodynamics of Engineering System Week 13 Chapter 10 Vapor & Combined Power Cycles The Carnot vapor cycle The Carnot cycle is the most efficient cycle operating between two specified temperature limits but it is not a suitable model for power cycles. Because: • Process 1-2 Limiting the heat transfer processes to two-phase systems severely limits the maximum temperature that can be used in the cycle (374°C for water) • Process 2-3 The turbine cannot handle steam with a high moisture content because of the impingement of liquid droplets on the turbine blades causing erosion and wear. • Process 4-1 It is not practical to design a compressor that handles two phases. The cycle in (b) is not suitable since it requires isentropic compression to extremely high pressures and isothermal heat transfer at variable pressures. 1-2 isothermal heat addition in a boiler 2-3 isentropic expansion in a turbine 3-4 isothermal heat rejection in a condenser 4-1 isentropic compression in a compressor T-s diagram of two Carnot vapor cycles. 1 Rankine cycle: The ideal cycle for vapor power cycles •Many of the impracticalities associated with the Carnot cycle can be eliminated by superheating the steam in the boiler and condensing it completely in the condenser. •The cycle that results is the Rankine cycle, which is the ideal cycle for vapor power plants. The ideal Rankine cycle does not involve any internal irreversibilities. Notes: • Only slight change in water temp through pump • The steam generator consists of boiler (two-phase heat transfer) and superheater • Turbine outlet is high-quality steam • Condenser is cooled by water (eg. -
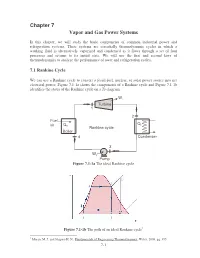
Chapter 7 Vapor and Gas Power Systems
Chapter 7 Vapor and Gas Power Systems In this chapter, we will study the basic components of common industrial power and refrigeration systems. These systems are essentially thermodyanmic cycles in which a working fluid is alternatively vaporized and condensed as it flows through a set of four processes and returns to its initial state. We will use the first and second laws of thermodynamics to analyze the performance of ower and refrigeration cycles. 7.1 Rankine Cycle We can use a Rankine cycle to convert a fossil-fuel, nuclear, or solar power source into net electrical power. Figure 7.1-1a shows the components of a Rankine cycle and Figure 7.1-1b identifies the states of the Rankine cycle on a Ts diagram. Wt Turbine 1 2 Fuel QC air QH Rankine cycle Boiler 4 Condenser 3 Wp Pump Figure 7.1-1a The ideal Rankine cycle Figure 7.1-1b The path of an ideal Rankine cycle1 1 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 395 7-1 In the ideal Rankine cycle, the working fluid undergoes four reversible processes: Process 1-2: Isentropic expansion of the working fluid from the turbine from saturated vapor at state 1 or superheated vapor at state 1’ to the condenser pressure. Process 2-3: Heat transfer from the working fluid as it flows at constant pressure through the condenser with saturated liquid at state 3. Process 3-4: Isentropic compression in the pump to state 4 in the compressed liquid region. Process 4-1: Heat transfer to the working fluid as it flows at constant pressure through the boiler to complete the cycle. -

Comparison Between Existing Rankine Cycle Refrigeration Systems and Hygroscopic Cycle Technology (HCT) †
Comparison between existing Rankine Cycle refrigeration systems and Hygroscopic Cycle Technology (HCT) † Francisco Javier Rubio Serrano 1, Fernando Soto Pérez 2 *, Antonio J. Gutiérrez Trashorras 3, Guillermo Ausin Abad 4 1IMASA, Ingeniería y Proyectos, S.A., Carpinteros 12, 28670 Villaviciosa de Odón, Madrid, Spain, [email protected] 2IMASA, Ingeniería y Proyectos, S.A., Palacio Valdés 1, 33002, Oviedo, Asturias, Spain, [email protected] 3 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] 4 Energy Department, Escuela Politécnica de Ingeniería, Edificio de Energía, Universidad de Oviedo, 33203 Gijón, Asturias, Spain, [email protected] * Corresponding Author e-mail: [email protected] ; Tel.: +34-696-467-104 † Presented at IRCSEEME, Universidad de Oviedo, Mieres del Camino, Asturias, España, 2018. Abstract: The objective of this paper is to review the different cooling systems that can be used in a Rankine cycle, especially the new technology called HCT (Hygroscopic Cycle Technology), that is based on the physical and chemical principles of absorption machines to increase the Rankine cycle net electrical efficiency and improve the cooling conditions. This technology allows an efficient and economical condensation of exhaust steam at the outlet of the steam turbine and significant decreases the water consumption. Advantages and high potential of HCT for power plants are analysed, comparing it with the current refrigeration systems. Also performances, investment and operation costs for each of the systems, are studied. Keywords: Refrigeration, Evaporative, Condenser, Hygroscopic, Absorber, Performance, Water, Dry-cooling, Rankine, Power. 1 1. Introduction Development of industries and increasing of population have produced a huge Energy demand.