Effect of Gland Extracts on Digging and Residing Preferences of Red Imported fire Ant Workers (Hymenoptera: Formicidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Field Ecology 2009
Journal of Field Ecology 2009 FOREST DYNAMICS Species-independent, Scale-invariant self-similarity in the Daniel Poon 7 allometry of branches and trunks within a heterogeneous forest Disturbance and fire refugia: deviations from invariant scaling Jane Remfert 14 relations across plant communities Looking for a DBH power function in Cassandra Bog Woods Elizabeth Hood 20 Testing for self thinning in oaks and comparing results in the Melissa Brady 25 presence of Gayluccacia Biases in spatial sampling for size frequency distributions Theresa Ong 31 Neutral theory and the predictions of the species distribution of an Melissa Brady 43 edge habitat Seedling establishment over a disturbance gradient exposes a Daniel Poon 47 species dominance shift within an oak-hickory forest SPATIAL PATTERN FORMATION Examining ant mosaic in the Big Woods of the E.S. George Hyunmin Han 55 Reserve Adventures in self-organization: spatial distribution of Japanese Amanda Grimm 63 Barberry in the E.S. George Reserve Distribution of Sarracenia purpurea clusters in Hidden Lake Hyunmin Han 68 Bog of the E.S. George Reserve BOTANY A survey of host-liana relationships in a Michigan oak-hickory Jane Remfert 74 forest: specificity and overwhelmedness Vine distribution and colonization preferences in the Big Woods Alexa Unruh 81 Examining the relationship between growth and reproduction in Megan Banka 88 perennial forbs Ramets and rhizomes: trade-offs in clonal plants in relation to Elizabeth Hood 96 water availability Hybridization among Quercus veluntina and Quercus rubra is Semoya Phillips 100 evident but a pattern of organization is not COMMUNITY COMPOSITION The halo of life: patterns in ant species richness in a Michigan Leslie McGinnis 105 scrubland The effect of an environmental gradient on species abundance in Jane Skillman 111 Cassandra Bog Stands of Typha sp. -

Predicting Future Coexistence in a North American Ant Community Sharon Bewick1,2, Katharine L
Predicting future coexistence in a North American ant community Sharon Bewick1,2, Katharine L. Stuble1, Jean-Phillipe Lessard3,4, Robert R. Dunn5, Frederick R. Adler6,7 & Nathan J. Sanders1,3 1Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, Tennessee 2National Institute for Mathematical and Biological Synthesis, University of Tennessee, Knoxville, Tennessee 3Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark 4Quebec Centre for Biodiversity Science, Department of Biology, McGill University, Montreal, Quebec, Canada 5Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 6Department of Mathematics, University of Utah, Salt Lake City, Utah 7Department of Biology, University of Utah, Salt Lake City, Utah Keywords Abstract Ant communities, climate change, differential equations, mechanistic models, species Global climate change will remodel ecological communities worldwide. How- interactions. ever, as a consequence of biotic interactions, communities may respond to cli- mate change in idiosyncratic ways. This makes predictive models that Correspondence incorporate biotic interactions necessary. We show how such models can be Sharon Bewick, Department of Biology, constructed based on empirical studies in combination with predictions or University of Maryland, College Park, MD assumptions regarding the abiotic consequences of climate change. Specifically, 20742, USA. Tel: 724-833-4459; we consider a well-studied ant community in North America. First, we use his- Fax: 301-314-9358; E-mail: [email protected] torical data to parameterize a basic model for species coexistence. Using this model, we determine the importance of various factors, including thermal Funding Information niches, food discovery rates, and food removal rates, to historical species coexis- RRD and NJS were supported by DOE-PER tence. -

Radiation in Socially Parasitic Formicoxenine Ants
RADIATION IN SOCIALLY PARASITIC FORMICOXENINE ANTS DISSERTATION ZUR ERLANGUNG DES DOKTORGRADES DER NATURWISSENSCHAFTEN (D R. R ER . N AT .) DER NATURWISSENSCHAFTLICHEN FAKULTÄT III – BIOLOGIE UND VORKLINISCHE MEDIZIN DER UNIVERSITÄT REGENSBURG vorgelegt von Jeanette Beibl aus Landshut 04/2007 General Introduction II Promotionsgesuch eingereicht am: 19.04.2007 Die Arbeit wurde angeleitet von: Prof. Dr. J. Heinze Prüfungsausschuss: Vorsitzender: Prof. Dr. S. Schneuwly 1. Prüfer: Prof. Dr. J. Heinze 2. Prüfer: Prof. Dr. S. Foitzik 3. Prüfer: Prof. Dr. P. Poschlod General Introduction I TABLE OF CONTENTS GENERAL INTRODUCTION 1 CHAPTER 1: Six origins of slavery in formicoxenine ants 13 Introduction 15 Material and Methods 17 Results 20 Discussion 23 CHAPTER 2: Phylogeny and phylogeography of the Mediterranean species of the parasitic ant genus Chalepoxenus and its Temnothorax hosts 27 Introduction 29 Material and Methods 31 Results 36 Discussion 43 CHAPTER 3: Phylogenetic analyses of the parasitic ant genus Myrmoxenus 46 Introduction 48 Material and Methods 50 Results 54 Discussion 59 CHAPTER 4: Cuticular profiles and mating preference in a slave-making ant 61 Introduction 63 Material and Methods 65 Results 69 Discussion 75 CHAPTER 5: Influence of the slaves on the cuticular profile of the slave-making ant Chalepoxenus muellerianus and vice versa 78 Introduction 80 Material and Methods 82 Results 86 Discussion 89 GENERAL DISCUSSION 91 SUMMARY 99 ZUSAMMENFASSUNG 101 REFERENCES 103 APPENDIX 119 DANKSAGUNG 120 General Introduction 1 GENERAL INTRODUCTION Parasitism is an extremely successful mode of life and is considered to be one of the most potent forces in evolution. As many degrees of symbiosis, a phenomenon in which two unrelated organisms coexist over a prolonged period of time while depending on each other, occur, it is not easy to unequivocally define parasitism (Cheng, 1991). -

Borowiec Et Al-2020 Ants – Phylogeny and Classification
A Ants: Phylogeny and 1758 when the Swedish botanist Carl von Linné Classification published the tenth edition of his catalog of all plant and animal species known at the time. Marek L. Borowiec1, Corrie S. Moreau2 and Among the approximately 4,200 animals that he Christian Rabeling3 included were 17 species of ants. The succeeding 1University of Idaho, Moscow, ID, USA two and a half centuries have seen tremendous 2Departments of Entomology and Ecology & progress in the theory and practice of biological Evolutionary Biology, Cornell University, Ithaca, classification. Here we provide a summary of the NY, USA current state of phylogenetic and systematic 3Social Insect Research Group, Arizona State research on the ants. University, Tempe, AZ, USA Ants Within the Hymenoptera Tree of Ants are the most ubiquitous and ecologically Life dominant insects on the face of our Earth. This is believed to be due in large part to the cooperation Ants belong to the order Hymenoptera, which also allowed by their sociality. At the time of writing, includes wasps and bees. ▶ Eusociality, or true about 13,500 ant species are described and sociality, evolved multiple times within the named, classified into 334 genera that make up order, with ants as by far the most widespread, 17 subfamilies (Fig. 1). This diversity makes the abundant, and species-rich lineage of eusocial ants the world’s by far the most speciose group of animals. Within the Hymenoptera, ants are part eusocial insects, but ants are not only diverse in of the ▶ Aculeata, the clade in which the ovipos- terms of numbers of species. -

Hymenoptera: Formicidae) from Indomalaya and Australasia, with a Redescription of P
Zootaxa 4441 (1): 171–180 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4441.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:5F4989D0-B9A9-4830-8C60-A19A5575E9B9 Two new Prenolepis species (Hymenoptera: Formicidae) from Indomalaya and Australasia, with a redescription of P. dugasi from Vietnam JASON L. WILLIAMS1 & JOHN S. LAPOLLA2 1Entomology & Nematology Department, University of Florida, Gainesville, Florida, United States of America. E-mail: [email protected] 2Department of Biological Sciences, Towson University, Towson, Maryland, United States of America. E-mail: [email protected] Abstract Prenolepis is a lineage of formicine ants with its center of diversity in the Old World tropics. Three more Prenolepis spe- cies are added to the Indomalayan and Australasian fauna and another is synonymized, bringing the total number of Pre- nolepis species worldwide to 19. Two new species are described: P. nepalensis from Nepal and P. lakekamu from Papua New Guinea, the latter being the first in the genus east of Wallace’s Line. Additionally, P. dugasi Forel (comb. rev.) from Vietnam is transferred from Nylanderia and redescribed. Based on morphology, each of the three species appears to be most closely-related to other species found predominantly in or nearest to their respective bioregions: P. nepalensis most resembles P. darlena, P. fisheri, and P. fustinoda; P. lakekamu bears strongest resemblance to P. jacobsoni, P. jerdoni, and P. subopaca; and P. dugasi most resembles P. melanogaster. Descriptions, illustrations and images are provided for all three species. -

Rossomyrmex, the Slave-Maker Ants from the Arid Steppe Environments
Hindawi Publishing Corporation Psyche Volume 2013, Article ID 541804, 7 pages http://dx.doi.org/10.1155/2013/541804 Review Article Rossomyrmex, the Slave-Maker Ants from the Arid Steppe Environments F. Ruano,1 O. Sanllorente,1,2 A. Lenoir,3 and A. Tinaut1 1 Departamento de Zoolog´ıa, Universidad de Granada, 18071 Granada, Spain 2 Departamento de Biolog´ıa Experimental, Facultad de Ciencias Experimentales, Universidad de Jaen,´ Campus Las Lagunillas s/n, 23071 Jaen,´ Spain 3 Institut de Recherche sur la Biologie de l’Insecte, IRBI-UMR CNRS 7261, Faculte´ des Sciences et Techniques, UniversiteFranc´ ¸ois Rabelais, 37200 Tours, France Correspondence should be addressed to F. Ruano; [email protected] Received 8 March 2013; Accepted 9 May 2013 Academic Editor: David P. Hughes Copyright © 2013 F. Ruano et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The host-parasite genera Proformica-Rossomyrmex present four pairs of species with a very wide range of distribution from China to Southeastern Spain, from huge extended plains to the top of high mountains. Here we review (1) the published data on these pairs in comparison to other slave-makers; (2) the different dispersal ability in hosts and parasites inferred from genetics (chance of migration conditions the evolutionary potential of the species); (3) the evolutionary potential of host and parasite determining the coevolutionary process in each host-parasite system that we treat to define using cuticular chemical data. We find a lower evolutionary potential in parasites than in hosts in fragmented populations, where selective pressures give advantage to a limited female parasite migration due to uncertainty of locating a host nest. -

Arthropods of Public Health Significance in California
ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA California Department of Public Health Vector Control Technician Certification Training Manual Category C ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA Category C: Arthropods A Training Manual for Vector Control Technician’s Certification Examination Administered by the California Department of Health Services Edited by Richard P. Meyer, Ph.D. and Minoo B. Madon M V C A s s o c i a t i o n of C a l i f o r n i a MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA 660 J Street, Suite 480, Sacramento, CA 95814 Date of Publication - 2002 This is a publication of the MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA For other MVCAC publications or further informaiton, contact: MVCAC 660 J Street, Suite 480 Sacramento, CA 95814 Telephone: (916) 440-0826 Fax: (916) 442-4182 E-Mail: [email protected] Web Site: http://www.mvcac.org Copyright © MVCAC 2002. All rights reserved. ii Arthropods of Public Health Significance CONTENTS PREFACE ........................................................................................................................................ v DIRECTORY OF CONTRIBUTORS.............................................................................................. vii 1 EPIDEMIOLOGY OF VECTOR-BORNE DISEASES ..................................... Bruce F. Eldridge 1 2 FUNDAMENTALS OF ENTOMOLOGY.......................................................... Richard P. Meyer 11 3 COCKROACHES ........................................................................................... -

Cataglyphis Desert Ants: a Good Model for Evolutionary Biology in Darwin's
Cataglyphis desert ants: a good model for evolutionary biology in Darwin’s anniversary year—A review ALAIN LENOIR,1 SERGE ARON,2 XIM CERDÁ,3 AND ABRAHAM HEFETZ4 1IRBI, UMR CNRS 6035, Université François Rabelais, Faculté des Sciences, 37200 Tours, France. E-mail: [email protected] 2Université Libre de Bruxelles, Service Évolution Biologique & Écologie, C.P. 160/12 50, av. F.D. Roosevelt, 1050 Bruxelles, Belgique. E-mail: [email protected] 3Estación Biológica de Doñana, CSIC, Avda. Américo Vespucio s/n, E-41092 Sevilla, Spain. E-mail: [email protected] 4Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel. E-mail: [email protected] ABSTRACT Cataglyphis ants comprise one of the most characteristic groups of insects in arid regions around the Mediterranean basin and have been intensively stud- ied over the last 30 years. These ants are central-place foragers and scaven- gers, single-prey loaders that have become a model for insect navigation using sophisticated visual orientation, having lost pheromone orientation. They are highly heat-tolerant ants that forage close to their critical thermal limit dur- ing the hottest hours of the day, with their long-chain cuticular hydrocarbons protecting them from desiccation. This is exemplified in two Cataglyphis species, each of which developed different mechanisms for counteracting extreme heat when foraging: polymorphism of workers vs. physiological and behavioral adaptations. Several species in this genus have also become a model for studying nestmate recognition mechanisms. The role of cuticular hydrocarbons and the postpharyngeal gland as a reservoir of hydrocarbons in nestmate recognition was initially discovered mainly in Cataglyphis, includ- ing the first experimental demonstration of the Gestalt model of nestmate recognition. -
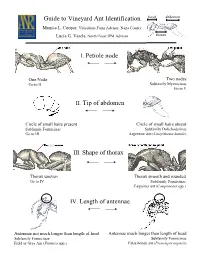
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

Pollination Biology of Ailanthus Altissima (Mill.) Swingle (Tree-Of-Heaven) in the Mid- Atlantic United States
Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science in Life Sciences In Entomology Richard D. Fell Carlyle C. Brewster P. Lloyd Hipkins R. Jay Stipes May 6, 2008 Blacksburg, Virginia Keywords: pollination, Ailanthus altissima, tree-of-heaven, nectar Copyright 2008 Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson ABSTRACT To date little information has been collected on the pollination biology of Ailanthus altissima (Mill.) Swingle (tree-of-heaven), an invasive exotic in the U.S. This study was conducted to determine the insect pollinator fauna visiting A. altissima and to study general pollinator visitation patterns associated with the tree’s nectar profile. A list of taxa visiting trees within each of three sites was developed from collected insects. Overall, visitor assemblage was dominated by the soldier beetle Chauliognathus marginatus with large numbers of ants in the genera Formica, Prenolepis, and Camponotus. No major diurnal pattern was found for visitation of insect pollinators using instantaneous counts. The nectar composition, concentration, and amount of total sugars in the flowers of A. altissima and how these are related to tree gender and time of day were determined. Nectar was found to be sucrose-dominant with lower, but nearly equal amounts of fructose and glucose. Total amounts of sugar in male and female blossoms were not statistically different, however higher concentrations of sugar were found in males (40.7%) than in females (35.3%). -

Author's Personal Copy
Author's personal copy Ant, Bee and Wasp Social Evolution R. Gadagkar, Indian Institute Science, Bangalore, India ã 2010 Elsevier Ltd. All rights reserved. Introduction and Definitions nest building, brood care, and colony maintenance, are performed by the workers, and this leads to a society of Ants, bees, and wasps belong to the Hymenoptera, a 220- female subjects headed by a female monarch. My-old monophyletic order, which is among the largest and most diverse in the class Insecta (Figure 1). Among the characters that are common to all Hymenoptera, The Paradox of Altruism perhaps the one that is of greatest interest to the topic of this article (even if it eventually turns out to be only of Queens mate and lay both haploid, unfertilized eggs and historical interest) is haplodiploidy, a term that indicates diploid, fertilized eggs. Workers are, by and large, sterile. In that males are haploid on account of developing parthe- some ant genera, such as , , and nogenetically from unfertilized eggs and females are dip- Pheidole Pheidologeton Sole- nopsis, workers have altogether lost their ovaries. In others, loid on account of developing from fertilized eggs. In spite of the size of this order (more than 250 000 described they have much smaller ovaries compared to their queens and in some species, workers have lost the ability to mate. species), only a small fraction (<10%) of the species is social, and thus of interest to us here. Even among those In any case, workers never or very rarely lay eggs when that are social, there is a very large variation in the degree their queen is alive so that they usually spend their whole lives working to assist their queen to reproduce, while of sociality. -

Distinct Chemical Blends Produced by Different Reproductive Castes in the Subterranean Termite Reticulitermes Flavipes
www.nature.com/scientificreports OPEN Distinct chemical blends produced by diferent reproductive castes in the subterranean termite Reticulitermes favipes Pierre‑André Eyer*, Jared Salin, Anjel M. Helms & Edward L. Vargo The production of royal pheromones by reproductives (queens and kings) enables social insect colonies to allocate individuals into reproductive and non‑reproductive roles. In many termite species, nestmates can develop into neotenics when the primary king or queen dies, which then inhibit the production of additional reproductives. This suggests that primary reproductives and neotenics produce royal pheromones. The cuticular hydrocarbon heneicosane was identifed as a royal pheromone in Reticulitermes favipes neotenics. Here, we investigated the presence of this and other cuticular hydrocarbons in primary reproductives and neotenics of this species, and the ontogeny of their production in primary reproductives. Our results revealed that heneicosane was produced by most neotenics, raising the question of whether reproductive status may trigger its production. Neotenics produced six additional cuticular hydrocarbons absent from workers and nymphs. Remarkably, heneicosane and four of these compounds were absent in primary reproductives, and the other two compounds were present in lower quantities. Neotenics therefore have a distinct ‘royal’ blend from primary reproductives, and potentially over‑signal their reproductive status. Our results suggest that primary reproductives and neotenics may face diferent social pressures.