Predicting Future Coexistence in a North American Ant Community Sharon Bewick1,2, Katharine L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Field Ecology 2009
Journal of Field Ecology 2009 FOREST DYNAMICS Species-independent, Scale-invariant self-similarity in the Daniel Poon 7 allometry of branches and trunks within a heterogeneous forest Disturbance and fire refugia: deviations from invariant scaling Jane Remfert 14 relations across plant communities Looking for a DBH power function in Cassandra Bog Woods Elizabeth Hood 20 Testing for self thinning in oaks and comparing results in the Melissa Brady 25 presence of Gayluccacia Biases in spatial sampling for size frequency distributions Theresa Ong 31 Neutral theory and the predictions of the species distribution of an Melissa Brady 43 edge habitat Seedling establishment over a disturbance gradient exposes a Daniel Poon 47 species dominance shift within an oak-hickory forest SPATIAL PATTERN FORMATION Examining ant mosaic in the Big Woods of the E.S. George Hyunmin Han 55 Reserve Adventures in self-organization: spatial distribution of Japanese Amanda Grimm 63 Barberry in the E.S. George Reserve Distribution of Sarracenia purpurea clusters in Hidden Lake Hyunmin Han 68 Bog of the E.S. George Reserve BOTANY A survey of host-liana relationships in a Michigan oak-hickory Jane Remfert 74 forest: specificity and overwhelmedness Vine distribution and colonization preferences in the Big Woods Alexa Unruh 81 Examining the relationship between growth and reproduction in Megan Banka 88 perennial forbs Ramets and rhizomes: trade-offs in clonal plants in relation to Elizabeth Hood 96 water availability Hybridization among Quercus veluntina and Quercus rubra is Semoya Phillips 100 evident but a pattern of organization is not COMMUNITY COMPOSITION The halo of life: patterns in ant species richness in a Michigan Leslie McGinnis 105 scrubland The effect of an environmental gradient on species abundance in Jane Skillman 111 Cassandra Bog Stands of Typha sp. -

The Ants of Oklahoma Master of Science
THE ANTS OF OKLAHOMA By Jerry H. Young(I\" Bachelor of Science Oklahoma Agricultural and Mechanical College Stillwater, Oklahoma 1955 Submitted to the faculty of the Graduate School of the Oklahoma Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE January 1 1956 tl<lAWMA AGCMCl«.f�Al L �Ci'!AlttCAl e&U.Ull LIBRARY JUL16195 6 THE ANTS OF OKLAHOMA Thesis Approved: Thesis Adviser }>JcMem��f � 't'" he Thesis ) Committee Member of the Thesis Committee 7'4'.��Member of the Thesis Committee Head of the Department ifean of the Graduate School 361565 ii PREFACE The study of the distribution of ants in the United States has been a long and continuous process with many contributors, but the State of Oklahoma has not received the attentions of these observers to any great extent. The only known list of ants of Oklahoma is one prepared by Mo Ro Smith (1935)0 Early in 1954 a survey of the state of Oklahoma was made to determine the species present and their distributiono The results of this survey, which blanketed the entire State, are given in this paper. The author wishes to express his appreciation to Dro Do E. Howell, chairman of the writer's thesis committee, for his valuable assistance and careful guidance in the preparation of this papero Also, much guidance on preparation of this manuscrip_t was received from Drs. Do Eo Bryan, William H. Irwin and F. A. Fenton. Many of the determin ations were made by M. R. Smith.. Vital infonnation was obtained from the museums at Oklahoma Agricultural and Mechanical College and the University of Oklahoma. -

Hymenoptera: Formicidae)
SYSTEMATICS Phylogenetic Analysis of Aphaenogaster Supports the Resurrection of Novomessor (Hymenoptera: Formicidae) 1 B. B. DEMARCO AND A. I. COGNATO Department of Entomology, Michigan State University, 288 Farm Lane, East Lansing, MI 48824. Ann. Entomol. Soc. Am. 108(2): 201–210 (2015); DOI: 10.1093/aesa/sau013 ABSTRACT The ant genus Aphaenogaster Mayr is an ecologically diverse group that is common throughout much of North America. Aphaenogaster has a complicated taxonomic history due to variabil- ity of taxonomic characters. Novomessor Emery was previously synonymized with Aphaenogaster, which was justified by the partial mesonotal suture observed in Aphaenogaster ensifera Forel. Previous studies using Bayesian phylogenies with molecular data suggest Aphaenogaster is polyphyletic. Convergent evolution and retention of ancestral similarities are two major factors contributing to nonmonophyly of Aphaenogaster. Based on 42 multistate morphological characters and five genes, we found Novomessor more closely related to Veromessor Forel and that this clade is sister to Aphaenogaster. Our results confirm the validity of Novomessor stat. r. as a separate genus, and it is resurrected based on the combi- nation of new DNA, morphological, behavioral, and ecological data. KEY WORDS Aphaenogaster, Novomessor, phylogenetics, resurrection Introduction phylogenetic analyses resolved Aphaenogaster as polyphyletic, including Messor Forel, 1890 and Sten- The ant genus Aphaenogaster Mayr, 1853 is a speciose amma (Brady et al. 2006, Moreau and Bell 2013). group,whichhasnotbeentaxonomicallyreviewedin Ward (2011) suggested that convergent evolution and over 60 years (Creighton 1950). Aphaenogaster con- retention of ancestral similarities were two major fac- tains 227 worldwide species (Bolton 2006), with 23 tors contributing to polyphyly of Aphaenogaster. valid North American species reduced from 31 original Aphaenogaster taxonomy was further complicated species descriptions. -

Hymenoptera: Formicidae) from Indomalaya and Australasia, with a Redescription of P
Zootaxa 4441 (1): 171–180 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4441.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:5F4989D0-B9A9-4830-8C60-A19A5575E9B9 Two new Prenolepis species (Hymenoptera: Formicidae) from Indomalaya and Australasia, with a redescription of P. dugasi from Vietnam JASON L. WILLIAMS1 & JOHN S. LAPOLLA2 1Entomology & Nematology Department, University of Florida, Gainesville, Florida, United States of America. E-mail: [email protected] 2Department of Biological Sciences, Towson University, Towson, Maryland, United States of America. E-mail: [email protected] Abstract Prenolepis is a lineage of formicine ants with its center of diversity in the Old World tropics. Three more Prenolepis spe- cies are added to the Indomalayan and Australasian fauna and another is synonymized, bringing the total number of Pre- nolepis species worldwide to 19. Two new species are described: P. nepalensis from Nepal and P. lakekamu from Papua New Guinea, the latter being the first in the genus east of Wallace’s Line. Additionally, P. dugasi Forel (comb. rev.) from Vietnam is transferred from Nylanderia and redescribed. Based on morphology, each of the three species appears to be most closely-related to other species found predominantly in or nearest to their respective bioregions: P. nepalensis most resembles P. darlena, P. fisheri, and P. fustinoda; P. lakekamu bears strongest resemblance to P. jacobsoni, P. jerdoni, and P. subopaca; and P. dugasi most resembles P. melanogaster. Descriptions, illustrations and images are provided for all three species. -

A Survey of the Ants (Hymenoptera: Formicidae) of Arkansas and the Ozark Mountains Joseph O'neill University of Arkansas, Fayetteville
University of Arkansas, Fayetteville ScholarWorks@UARK Horticulture Undergraduate Honors Theses Horticulture 12-2011 A Survey of the Ants (Hymenoptera: Formicidae) of Arkansas and the Ozark Mountains Joseph O'Neill University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/hortuht Recommended Citation O'Neill, Joseph, "A Survey of the Ants (Hymenoptera: Formicidae) of Arkansas and the Ozark Mountains" (2011). Horticulture Undergraduate Honors Theses. 1. http://scholarworks.uark.edu/hortuht/1 This Thesis is brought to you for free and open access by the Horticulture at ScholarWorks@UARK. It has been accepted for inclusion in Horticulture Undergraduate Honors Theses by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. A Survey of the Ants (Hymenoptera: Formicidae) of Arkansas and the Ozark Mountains An Undergraduate Honors Thesis at the University of Arkansas Submitted in partial fulfillment of the requirements for the University of Arkansas Dale Bumpers College of Agricultural, Food and Life Sciences Honors Program by Joseph C. O’Neill and Dr. Ashley P.G. Dowling December 2011 < > Dr. Curt R. Rom < > Dr. Ashley P.G. Dowling < > Dr. Donn T. Johnson < > Dr. Duane C. Wolf ABSTRACT Ants are among the most abundant animals in most terrestrial ecosystems, yet local fauna are often poorly understood due to a lack of surveys. This study separated and identified ant species from arthropod samples obtained during ongoing projects by the lab of Dr. A.P.G. Dowling, Professor of Entomology at the University of Arkansas. More than 600 ants were prepared, 284 of which were identified to genus and 263 to species. -

Arthropods of Public Health Significance in California
ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA California Department of Public Health Vector Control Technician Certification Training Manual Category C ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA Category C: Arthropods A Training Manual for Vector Control Technician’s Certification Examination Administered by the California Department of Health Services Edited by Richard P. Meyer, Ph.D. and Minoo B. Madon M V C A s s o c i a t i o n of C a l i f o r n i a MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA 660 J Street, Suite 480, Sacramento, CA 95814 Date of Publication - 2002 This is a publication of the MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA For other MVCAC publications or further informaiton, contact: MVCAC 660 J Street, Suite 480 Sacramento, CA 95814 Telephone: (916) 440-0826 Fax: (916) 442-4182 E-Mail: [email protected] Web Site: http://www.mvcac.org Copyright © MVCAC 2002. All rights reserved. ii Arthropods of Public Health Significance CONTENTS PREFACE ........................................................................................................................................ v DIRECTORY OF CONTRIBUTORS.............................................................................................. vii 1 EPIDEMIOLOGY OF VECTOR-BORNE DISEASES ..................................... Bruce F. Eldridge 1 2 FUNDAMENTALS OF ENTOMOLOGY.......................................................... Richard P. Meyer 11 3 COCKROACHES ........................................................................................... -
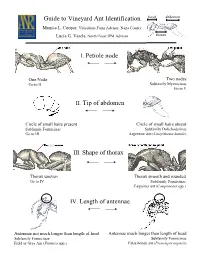
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

Pollination Biology of Ailanthus Altissima (Mill.) Swingle (Tree-Of-Heaven) in the Mid- Atlantic United States
Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science in Life Sciences In Entomology Richard D. Fell Carlyle C. Brewster P. Lloyd Hipkins R. Jay Stipes May 6, 2008 Blacksburg, Virginia Keywords: pollination, Ailanthus altissima, tree-of-heaven, nectar Copyright 2008 Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson ABSTRACT To date little information has been collected on the pollination biology of Ailanthus altissima (Mill.) Swingle (tree-of-heaven), an invasive exotic in the U.S. This study was conducted to determine the insect pollinator fauna visiting A. altissima and to study general pollinator visitation patterns associated with the tree’s nectar profile. A list of taxa visiting trees within each of three sites was developed from collected insects. Overall, visitor assemblage was dominated by the soldier beetle Chauliognathus marginatus with large numbers of ants in the genera Formica, Prenolepis, and Camponotus. No major diurnal pattern was found for visitation of insect pollinators using instantaneous counts. The nectar composition, concentration, and amount of total sugars in the flowers of A. altissima and how these are related to tree gender and time of day were determined. Nectar was found to be sucrose-dominant with lower, but nearly equal amounts of fructose and glucose. Total amounts of sugar in male and female blossoms were not statistically different, however higher concentrations of sugar were found in males (40.7%) than in females (35.3%). -

Nutritional Ecology of Aphaenogaster Ants in Response to Climate Change Katie A
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2018 Nutritional Ecology of Aphaenogaster Ants in Response to Climate Change Katie A. Miller University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Ecology and Evolutionary Biology Commons Recommended Citation Miller, Katie A., "Nutritional Ecology of Aphaenogaster Ants in Response to Climate Change" (2018). Graduate College Dissertations and Theses. 899. https://scholarworks.uvm.edu/graddis/899 This Thesis is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. NUTRITIONAL ECOLOGY OF APHAENOGASTER ANTS IN RESPONSE TO CLIMATE CHANGE A Thesis Presented by Katie Ann Miller to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Master of Science Specializing in Biology May, 2018 Defense Date: March 21st, 2018 Thesis Examination Committee: Sara Helms Cahan, Ph.D., Advisor Kimberly Wallin, Ph.D., Chairperson Nicholas Gotelli, Ph.D. Jason Stockwell, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT Climate change is predicted to impact organismal nutritional ecology. Increased temperatures can directly accelerate physiological rate processes, which in turn, impact nutritional requirements. Climate change can also impact organisms indirectly by altering the quality and quantity of nutritional resources, creating the potential for nutritional mismatch between what nutrients are available in the environment and what organisms require. -

Nylanderia (=Paratrechina) Pubens (Forel) (Insecta: Hymenoptera: Formicidae: Formicinae)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY284 Caribbean Crazy Ant (proposed common name), Nylanderia (=Paratrechina) pubens (Forel) (Insecta: Hymenoptera: Formicidae: Formicinae)1 John Warner and Rudolph H. Scheffrahn2 Introduction Samples of N. pubens collected in Coral Gables and Miami, Florida, date from 1953 (Trager 1984). Beginning about 2000, reports have escalated of Klotz et al. (1995) report infestations in Boca Raton, a golden-brown to reddish-brown "crazy ant" Homestead, and Miami and state that "in 1990, infesting properties in and around West Palm Beach, hundreds of these ants were found on the second Florida. Thick foraging trails with thousands of ants floor of a large Miami hospital." Deyrup et al. (2000) occur along sidewalks, around buildings, and on trees report that it "is abundant on the campus of the and shrubs. Pest control operators using liquid and/or University of Miami, where it resembles a pale N. granular broad-range insecticides appear unable to bourbonica, foraging on sidewalks and running up control this nuisance ant. and down tree trunks." L. Davis, Jr. (2003 personal communication) has seen these ants from Everglades Synonymy National Park, Fort Lauderdale, Jacksonville, and Port St. Lucie. Specimens from Sarasota (F. Santana Paratrechina pubens Forel 1893 2003, personal communication) were also confirmed. Distribution These ants seem to have large populations where they occur and are considered a pest in Colombia (Davis Nylanderia pubens (Forel) was originally 2003, personal communication). described as Paratrechina pubens Forel from St. Vincent, Lesser Antilles, and has been found on other In the West Palm Beach area, two heavily West Indian islands, including Anguilla, Guadeloupe, infested sites were observed about 3 miles west of the and Puerto Rico (Trager 1984). -

Effect of Gland Extracts on Digging and Residing Preferences of Red Imported fire Ant Workers (Hymenoptera: Formicidae)
Insect Science (2013) 20, 456–466, DOI 10.1111/j.1744-7917.2012.01553.x ORIGINAL ARTICLE Effect of gland extracts on digging and residing preferences of red imported fire ant workers (Hymenoptera: Formicidae) Jian Chen and Guangmei Zhang USDA-ARS, National Biological Control Laboratory, Biological Control of Pests Research Unit, Stoneville, MS 38776, USA Abstract There is evidence that ant-derived chemical stimuli are involved in regulat- ing the digging behavior in Solenopsis invicta Buren. However, the source gland(s) and chemistry of such stimuli have never been revealed. In this study, extracts of mandibular, Dufour’s, postpharyngeal, and poison glands were evaluated for their effect on ant digging and residing preferences of S. invicta workers from three colonies. In the intracolonial bioassays, workers showed significant digging preferences to mandibular gland extracts in 2 of 3 colonies and significant residing preferences in 1 of 3 colonies; significant digging preferences to Dufour’sgland extracts in 1 of 3 colonies and significant residing preferences in 2 of 3 colonies. No digging and residing preferences were found for postpharyngeal and poison gland extracts. In intercolonial bioassays, significant digging and residing prefer- ences were found for mandibular gland extracts in 3 of 6 colony combinations. Significant digging preferences to Dufour’s gland extracts were found in 4 of 6 colony combinations and significant residing preferences in all 6 colony combinations. For postpharyngeal gland extracts, significant digging preferences were found only in 1 of 6 colonial combinations and no significant residing preferences were found. For poison gland extracts, no signif- icant digging preferences were found; significant residing preferences were found in 1 of 6 colony combinations. -

Environmental Variables Affecting Ant (Formicidae)
ENVIRONMENTAL VARIABLES AFFECTING ANT (FORMICIDAE) COMMUNITY COMPOSITION IN MISSISSIPPI’S BLACK BELT AND FLATWOODS REGIONS By JoVonn Grady Hill A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Agricultural Life Sciences with a Concentration in Entomology in the Department of Entomology and Plant Pathology Mississippi State University May 2006 ENVIRONMENTAL VARIABLES AFFECTING ANT (FORMICIDAE) COMMUNITY COMPOSITION IN MISSISSIPPI’S BLACK BELT AND FLATWOODS REGIONS. By JoVonn Grady Hill Approved: Richard L. Brown Keith Summerville Professor of Entomology Assistant Professor of Environmental (Director of Thesis) Science and Policy (Adjunct) Drake University, Des Moines, IA Committee Member ___________________________ C. Evan Peacock Clarence H. Collison Professor of Anthropology Graduate Coordinator (Committee Member) (Committee Member) Vance H. Watson Dean of the College of Agriculture and Life Sciences Name: JoVonn Grady Hill Date of Degree: May 13, 2006 Institution: Mississippi State University Major Field: Entomology Major Professor: Dr. Richard L. Brown Title of Study: ENVIRONMENTAL VARIABLES AFFECTING ANT (FORMICIDAE) COMMUNITY COMPOSITION IN MISSISSIPPI’S BLACK BELT AND FLATWOODS REGIONS Pages in Study: 72 Candidate for Degree of Master of Science The relationship of ant community composition to various habitat characteristics is compared across four habitat types and 12 environmental variables in Mississippi. The four habitat types include pasture, prairie, and oak-hickory forests in the Black Belt and forests in the Flatwoods physiographic region. Ants were sampled using pitfall traps, litter sampling, baiting and hand collecting. A total of 20,916 ants representing 68 species were collected. NMS and ANCOVA both revealed three distinct ant communities (pasture, prairie, and “forests”) based on species composition and mean ant abundance per habitat type between the four habitat types.