1 Chiral Analysis of Pollutants and Their Metabolites by Capillary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cypermethrin
International Environmental Health Criteria 82 Cypermethrin Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization WORLD HEALTH ORGANIZATION GENEVA 1989 Other titles available in the ENVIRONMENTAL HEALTH CRITERIA series include: 1. Mercury 2. Polychlorinated Biphenyls and Terphenyls 3. Lead 4. Oxides of Nitrogen 5. Nitrates, Nitrites, and N-Nitroso Compounds 6. Principles and Methods for Evaluating the Toxicity of Chemicals, Part 1 7. Photochemical Oxidants 8. Sulfur Oxides and Suspended Particulate Matter 9. DDT and its Derivatives 10. Carbon Disulfide 11. Mycotoxins 12. Noise 13. Carbon Monoxide 14. Ultraviolet Radiation 15. Tin and Organotin Compounds 16. Radiofrequency and Microwaves 17. Manganese 18. Arsenic 19. Hydrogen Sulfide 20. Selected Petroleum Products 21. Chlorine and Hydrogen Chloride 22. Ultrasound 23. Lasers and Optical Radiation 24. Titanium 25. Selected Radionuclides 26. Styrene 27. Guidelines on Studies in Environmental Epidemiology 28. Acrylonitrile 29. 2,4-Dichlorophenoxyacetic Acid (2,4-D) 30. Principles for Evaluating Health Risks to Progeny Associated with Exposure to Chemicals during Pregnancy 31. Tetrachloroethylene 32. Methylene Chloride 33. Epichlorohydrin 34. Chlordane 35. Extremely Low Frequency (ELF) Fields 36. Fluorine and Fluorides 37. Aquatic (Marine and Freshwater) Biotoxins 38. Heptachlor 39. Paraquat and Diquat 40. Endosulfan 41. Quintozene 42. Tecnazene 43. Chlordecone 44. Mirex continued on p. 156 -

Genetically Modified Baculoviruses for Pest
INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS This page intentionally left blank INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS EDITED BY LAWRENCE I. GILBERT SARJEET S. GILL Amsterdam • Boston • Heidelberg • London • New York • Oxford Paris • San Diego • San Francisco • Singapore • Sydney • Tokyo Academic Press is an imprint of Elsevier Academic Press, 32 Jamestown Road, London, NW1 7BU, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA ª 2010 Elsevier B.V. All rights reserved The chapters first appeared in Comprehensive Molecular Insect Science, edited by Lawrence I. Gilbert, Kostas Iatrou, and Sarjeet S. Gill (Elsevier, B.V. 2005). All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission in writing from the publishers. Permissions may be sought directly from Elsevier’s Rights Department in Oxford, UK: phone (þ44) 1865 843830, fax (þ44) 1865 853333, e-mail [email protected]. Requests may also be completed on-line via the homepage (http://www.elsevier.com/locate/permissions). Library of Congress Cataloging-in-Publication Data Insect control : biological and synthetic agents / editors-in-chief: Lawrence I. Gilbert, Sarjeet S. Gill. – 1st ed. p. cm. Includes bibliographical references and index. ISBN 978-0-12-381449-4 (alk. paper) 1. Insect pests–Control. 2. Insecticides. I. Gilbert, Lawrence I. (Lawrence Irwin), 1929- II. Gill, Sarjeet S. SB931.I42 2010 632’.7–dc22 2010010547 A catalogue record for this book is available from the British Library ISBN 978-0-12-381449-4 Cover Images: (Top Left) Important pest insect targeted by neonicotinoid insecticides: Sweet-potato whitefly, Bemisia tabaci; (Top Right) Control (bottom) and tebufenozide intoxicated by ingestion (top) larvae of the white tussock moth, from Chapter 4; (Bottom) Mode of action of Cry1A toxins, from Addendum A7. -
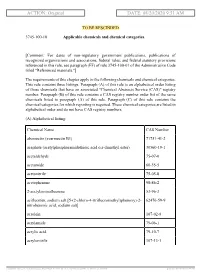
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -

(12) United States Patent (10) Patent No.: US 8,852,618 B2 Clough (45) Date of Patent: Oct
USOO8852618B2 (12) United States Patent (10) Patent No.: US 8,852,618 B2 Clough (45) Date of Patent: Oct. 7, 2014 (54) INSECTICIDAL MIXTURE CONTAINING CA 2429218 A1 6, 2002 GAMMA-CYHALOTHRN CH 689326 A5 4f1995 EP O237227 A1 9, 1987 EP 0771526 A2 5, 1997 (75) Inventor: Martin Stephen Clough, Bracknell EP O988788 A1 3f2000 (GB) FR 272O230 A1 12/1995 JP 63. 126805 A2 5, 1988 (73) Assignee: Syngenta Limited, Guildford (GB) JP 63126805 A2 5, 1988 JP 63126805 5, 1998 c - r WO WO 86 O7525 A1 12, 1986 (*) Notice: Subject to any disclaimer, the term of this WO WO 93 03618 A2 3, 1993 patent is extended or adjusted under 35 WO WO95 229O2 A1 8/1995 U.S.C. 154(b) by 824 days. WO WO9533380 A1 12, 1995 WO WO 96 16543 A2 6, 1996 (21) Appl. No.: 12/633,063 WO WO97 06687 A1 2/1997 WO WO974O692 A1 11, 1997 (22) Filed: Dec.a V88, 2009 WO WOOOO2453 A1 1, 2000 OTHER PUBLICATIONS (65) Prior Publication Data US 201O/OO81714 A1 Apr. 1, 2010 Canadian Office Action (Applin. No. 2,452,515 filed: Jul. 10, 2002) mailing date Oct. 1, 2010 (pp. 1-2). Related U.S. Application Data Allen et al. Transgenic & Conventional Insect & Weed Control Sys tems; Proceedings of the Beltwide Cotton Conference, vol. 2, 1065 (62) Division of application No. 10/484.745, filed as 1068 (1999), USA. application No. PCT/GB02/03181 on Jul. 10, 2002, Anonymous; Pesticide Mixtures for Control of Insect and Acarid now Pat. No. -

NASTTPO CFATS Information 2Nd Edition
National Association of SARA Title III Program Officials Concerned with the Emergency Planning and Community Right-to-Know Act January 13, 2008 Issue 07-01 2nd Edition The Department of Homeland Security has adopted 6 CFR Part 27, a new regulation mandated by Congress, known as Chemical Facility Anti-Terrorism Standards (CFATS). The regulation is intended to fill a security gap in our country’s anti-terrorism efforts by identifying and improving the security of chemicals that are potentially at a high level of risk for release, theft, or sabotage. LEPCs and SERCs should alert EPCRA & RMP reporting facilities about these requirements. No reports are due to the LEPCs and SERCs under these requirements; however, given the potential for security requirements to have an impact on facility access for emergency responders and on emergency plans, it is critical for local planners, responders and facilities to communicate in order for a facility to satisfy the regulatory requirements. In order to aid LEPCs, SERCs and facilities in understanding these new requirements we have prepared some short-hand aids. Following this cover page is a key issue comparison between EPCRA, RMP and the CFATS regulation. As requirements may change the user is counseled to look for updated information. Following the side-by-side comparison we have edited the EPA “List of Lists” to add the Appendix A list of chemicals and thresholds from the CFATS program. This list may change and we will update these materials when that happens. The initial requirement for a facility with an Appendix A chemical at or over the threshold is to submit a Top-Screen. -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

Annexure – List of Raw Materials (Agreement)
Annexure – List of Raw Materials (Agreement) Company will make agreement with supplier of raw material after getting EC & CTE. Some raw material will import and some will be local. Sr. Product Name Raw Material Consumption CAS NOS. LD 50 No. (MT/MT of product) (mg/kg) Existing Additional Total 1. 2-4-D Ethyl Ester 2,4-D acid 0.95 0 0.95 94-75-7 699 Ethyl alcohol 0.21 0 0.21 64-17-5 140 2. 2-4-D Sodium Salt 2,4 –Dichloro phenol 0.725 0 0.725 120-83-2 47 Mono Chloro Acetic Acid 0.520 0 0.520 79-11-8 165 3. Abamectin Streptomycessavermitis 0. 550 0 0. 550 NA NA Anthelminic 1.100 0 1.100 NA NA Acaricidal 1.100 0 1.100 NA NA 4. Acetamiprid N-Cyano methyl Acetamidate 0. 500 0 0. 500 5652 -84 -6 NA 2-Chloro 5-(methyl amino methyl) Pyridine 0.730 0 0.730 18368-64-4 NA Methanol 0.200 0 0.200 67-56-1 5628 5. Allethrin Cyclohexane 0. 930 0 0. 930 110 -82 -7 39 Allethrolone 0. 540 0 0. 540 29605 -88 -7 NA Pyridine 0.350 0 0.350 110-86-1 891 Chrysanthemic acid chloride 0.640 0 0.640 4638-92-0 NA 6. Alpha Cypermethrin Meta phenoxy benzaldehyde 0.714 0 0.714 39515-51-0 1222 Sodium cyanide 0.195 0 0.195 143-33-9 6.440 n- Hexane 4.300 0 4.300 110 -54 -3 5000 Cyprmethric acid chloride 0.835 0 0.835 52314-67-7 NA 7. -

Title Studies on Saligenin Cyclic Phosphorus Esters with Insecticidal
Studies on Saligenin Cyclic Phosphorus Esters with Title Insecticidal Activity : Part X. Synergism of Malathion against Susceptible and Resistant Insects ETO, Morifusa; OSHIMA, Yasuyoshi; KITAKATA, Setuo; Author(s) TANAKA, Fumikazu; KOJIMA, Ken'ichi Citation 防虫科学 (1966), 31(1): 33-38 Issue Date 1966-02-28 URL http://hdl.handle.net/2433/158466 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University m m fl ~ in 31 ~-I ~Wrff1lJ'Il:ar1tl-f-~ 24n~mO~0)~¥E~~l2;.R~t.:. c z hl2 Summary Bliss 0) probit r.tI~ t...t.:t.I~., -cmJ.1nt.... -( zcr:»: 1 iiJi Exploratory studies have been made with ~ I) O)rf:t~~r~rln1lt7Ja.Frf1~~3'Er!ftl:l2;J<~t.:. substituted benzyl esters of chrysanthemic acid M* :~~O)M*l2;U~'r Q c Table 1. sos 2 0) in search for effective insecticides. Of many benzyl tm< '"C"<b:Q. chrysanthemates synthesized, 4-allylbenzyl chry santhemate was found to show a high insecticidal activity and was designated as "Benathrin". 1) Chen, Y. L., Barthel. W. F. : U. 5. Dept. Agri., Benathrin was as 1.7 times toxic in knock·down ARS 23 (1956). and 9.2 times toxic in mortality as a-til-trans 2) Piquett, P. G.. Gersdorff, W. A.: J. Econ, allethrin to adult house fly, Musca domestica Entomol.• 51. 791 (1958). vicina Maq., when applied topically in acetone 3) Buei, K.: Boiyu-kagaku, 30, 37 (1965). solution. Studies on Saligenin Cyclic Phosphorus Esters with .Insecticidal Activity. Part X. Synergism of Malathion against Susceptible and Resistant Insects. Morifusa ETO, Yasuyoshi Osmsr», Setuo KITAKATA*. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Risk Assessment 7.1.1 Introduction As Indicated in the Earlier Chapter The
Environmental Impact Assessment Report For Expansion of M/s Shogun Organics Ltd. Located At Kurkumbh MIDC, Taluka Daund, District Pune, Maharashtra. SEEPL/EIA/F/IND/SOL/001/2017-18/00 Chapter 7: Additional Studies Risk Assessment 7.1.1 Introduction As indicated in the earlier chapter the expansion project facilitates to manufacture pesticide, pesticide intermediates and related formulations various types of hazards due to storage, handling & transportation of various chemicals, which are indicated in Chapter no. 2. In order to study the risks envisaged by the activities, the following methodology was adopted. 7.1.1.1 The factory would be manufacturing various products with production quantity Pesticide Technical – 44.5 MT/M, Intermediates – 12.25 MT/M, By-products – 13.8 MT/M and heater machines – 25,000 nos./month. The products would be manufactured on batch basis; a maximum of five products will be manufactured at any given point of time. However hazop studies have been carried out for some hazardous operations and process. 7.1.1.2 A detailed scrutiny of various chemicals which would be stored on the site was done. All the raw materials will be stored either in bags/drums/boxes or carbuoy. Details of the raw materials in storage area is tabulated in Table no – 7.1. 7.1.1.3 Plot layouts showing locations of tanks, fire hydrant tanks & other safety features are depicted in the plot layout discussed in the onsite emergency plan. 7.1.1.4 M.S.D.S.s: Brief M.S.D.S.s of all the above chemicals and other raw materials which would be used in the manufacturing processes of products are used as reference for preparation of Quantitative Risk Assessment & Hazop study.The copies of MSDS is attached as Annexure – 7.5. -

Pyrethroids: How They Affect Human and Animal Health?
medicina Editorial Pyrethroids: How They Affect Human and Animal Health? Iga Hoły ´nska-Iwan 1,* and Karolina Szewczyk-Golec 2 1 Laboratory of Electrophysiology of Epithelial Tissue and Skin, Department of Pathobiochemistry and Clinical Chemistry, Faculty of Pharmacy, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, 87-100 Torun, Poland 2 Department of Medical Biology and Biochemistry, Faculty of Medicine, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, 87-100 Torun, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-525853598 Received: 21 October 2020; Accepted: 29 October 2020; Published: 30 October 2020 Abstract: Pyrethroids are pesticides commonly used in crop protection; in the forestry, wood, and textile industries; as well as in medicine and veterinary medicine to treat parasitic crustacean infestations. They have been found to be relatively safe for humans and animals. Pyrethroids are recommended for personal protection against malaria and virus Zika by the World Health Organization. Pyrethroids act on voltage-gated sodium channels, which cause an influx of sodium ions into the nerve cells and permanent depolarization. They also influence activities of enzymes, especially in nerve and liver cells. Contact of pyrethroids with the skin, digestive tract, and respiratory tract results in their penetration into the body. Due to the importance of the subject, a summary of the current state of knowledge on the toxic effects of pyrethroids was presented in the comprehensive review by Chrustek et al, published in journal Medicina. Particular attention was paid to nephrotoxic, hepatotoxic, cardiotoxic, immunotoxic, neurotoxic, and behavioral effects of pyrethroids on human and animal bodies. -

Pyrethoid Insecticides
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California 94025 Abstract Process Economics Program Report No. 143 PYRETHROID INSECTICIDES (June 1981) The technology, economics, and marketing strategies of synthetic pyrethroids are reviewed. Four relatively new compounds--permethrin, cypermethrin, decamethrin, and fenvalerate--are being commercially used in controlling many agricultural insect pests at practical doses up to one-tenth that required by conventional synthetic insecticides, with significantly lower polluting persistence, lower mammalian toxicity, and lower insect resistance. The technology consists of a series of complex stepwise organic syntheses, most of which are covered by recent patents issued to many companies which are already active in or planning to enter this field. An in-depth technical and economic analysis is made for a perme- thrin design at 2 million lb per year with educated cost approximations for the other compounds. The processes are based upon the use of rela- tively inexpensive raw materials to produce all the required intermedi- ates rather than purchase these intermediates at expensive prices. PEP'79 LAW For detailed marketing data and information, the reader is referred to one of the SRI programs specializing in marketing research. The CHEMICAL ECONOMICS HANDBOOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTORY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION. 1 2 SUMMARY .......................... 3 General ........................... 3 Marketers .......................... 6 Applications.