Mechanistic Studies on Medium Chain Acyl-Coa Dehydrogenase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inborn Errors of Metabolism Test Requisition
LABORATORY OF GENETICS AND GENOMICS Mailing Address: For local courier service and/or inquiries, please contact 513-636-4474 • Fax: 513-636-4373 3333 Burnet Avenue, Room R1042 www.cincinnatichildrens.org/moleculargenetics • Email: [email protected] Cincinnati, OH 45229 INBORN ERRORS OF METABOLISM TEST REQUISITION All Information Must Be Completed Before Sample Can Be Processed PATIENT INFORMATION ETHNIC/RACIAL BACKGROUND (Choose All) Patient Name: ___________________ , ___________________ , ________ European American (White) African-American (Black) Last First MI Native American or Alaskan Asian-American Address: ____________________________________________________ Pacific Islander Ashkenazi Jewish ancestry ____________________________________________________ Latino-Hispanic _____________________________________________ Home Phone: ________________________________________________ (specify country/region of origin) MR# __________________ Date of Birth ________ / ________ / _______ Other ____________________________________________________ (specify country/region of origin) Gender: Male Female BILLING INFORMATION (Choose ONE method of payment) o REFERRING INSTITUTION o COMMERCIAL INSURANCE* Insurance can only be billed if requested at the time of service. Institution: ____________________________________________________ Policy Holder Name: _____________________________________________ Address: _____________________________________________________ Gender: ________________ Date of Birth ________ / ________ / _______ -

ACADS Rabbit Pab
Leader in Biomolecular Solutions for Life Science ACADS Rabbit pAb Catalog No.: A0945 2 Publications Basic Information Background Catalog No. This gene encodes a tetrameric mitochondrial flavoprotein, which is a member of the A0945 acyl-CoA dehydrogenase family. This enzyme catalyzes the initial step of the mitochondrial fatty acid beta-oxidation pathway. Mutations in this gene have been Observed MW associated with short-chain acyl-CoA dehydrogenase (SCAD) deficiency. Alternative 44kDa splicing results in two variants which encode different isoforms. Calculated MW 44kDa Category Primary antibody Applications WB, IHC, IF Cross-Reactivity Human, Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 35 P16219 IHC 1:50 - 1:100 Immunogen 1:50 - 1:200 IF Recombinant fusion protein containing a sequence corresponding to amino acids 1-260 of human ACADS (NP_000008.1). Synonyms ACADS;ACAD3;SCAD Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide,50% glycerol,pH7.3. Validation Data Western blot analysis of extracts of various cell lines, using ACADS antibody (A0945) at 1:1000 dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) (AS014) at 1:10000 dilution. Lysates/proteins: 25ug per lane. Blocking buffer: 3% nonfat dry milk in TBST. Detection: ECL Basic Kit (RM00020). Exposure time: 15s. Immunohistochemistry of paraffin- Immunohistochemistry of paraffin- Immunofluorescence analysis of U2OS embedded human liver cancer using embedded human liver damage using cells using ACADS antibody (A0945). Blue: ACADS antibody (A0945) at dilution of ACADS antibody (A0945) at dilution of DAPI for nuclear staining. -

Positive Carrier Sample Report
RESULTS RECIPIENT FEMALE MALE UNIVERSITY MEDICAL CENTER JANE MILLER N/A Attn: Dr. Paul Smith DOB: 11/11/1977 123 Main Street Ethnicity: Northern European City, CA 10231 Sample Type: OG-510 Saliva Phone: (800) 555-1212 Date of Collection: 02/06/2020 Fax: (800) 555-1212 Date Received: 02/16/2020 NPI: 4253506008 Date Tested: 02/16/2020 Report Date: 02/18/2020 Barcode: 55200006634190 Accession ID: FAKERQSCARFAF Indication: Screening for genetic disease carrier status Foresight® Carrier Screen POSITIVE: CARRIER ABOUT THIS TEST The Myriad Foresight Carrier Screen utilizes sequencing, maximizing coverage across all DNA regions tested, to help you learn about your chance to have a child with a genetic disease. RESULTS SUMMARY Risk Details JANE MILLER Partner Panel Information Foresight Carrier Screen N/A Universal Panel Fundamental Plus Panel Fundamental Panel Fragile X Syndrome (176 conditions tested) POSITIVE: CARRIER CARRIER* The reproductive risk presented Smith‑Lemli‑Opitz Syndrome NM_001360.2(DHCR7):c. is based on a hypothetical 964-1G>C(aka IVS8-1G>C) pairing with a partner of the Reproductive Risk: 1 in 380 heterozygote same ethnic group. Carrier Inheritance: Autosomal Recessive testing should be considered. See "Next Steps". *Carriers generally do not experience symptoms. No disease-causing mutations were detected in any other gene tested. A complete list of all conditions tested can be found on page 6. Additional Findings Single Carrier Autosomal recessive additional findings CLINICAL NOTES NEXT STEPS • None • Carrier testing should be considered for the diseases specified above for the patient's partner, as both parents must be carriers before a child is at high risk of developing the disease. -

Excluded Test List – As of 08/01/2016
Excluded Test List – as of 08/01/2016 CPT As Gene Code 81161 DMD, dup_del 81200 ASPA, cv 81201 APC, fgs 81202 APC, kfv 81203 APC, dup_del 81205 BCKDHA, cv 81205 BCKDHB, cv 81209 BLM, 2281del6ins7 81220 CFTR, cv 81221 CFTR, kfv 81222 CFTR, dup_del 81223 CFTR, fgs 81224 CFTR, intron 8 poly-T 81228 Microarray, CNV 81229 Microarray, SNP 81240 F2, 20210G>A 81241 F5, Leiden (1691G>A) 81242 FANCC, cv 81243 FMR1 81244 FMR1, ma 81250 G6PC, cv 81251 GBA, cv 81252 GJB2, fgs 81253 GJB2, kfv 81254 GJB6, cv 81255 HEXA, cv 81257 HBA1_HBA2, cdel or v 81260 IKBKAP, cv 81271 SRY-FISH 81280 LQT, fgs 12 genes 81281 LQT, kfv 81282 LQT, dup_del 12 genes 81290 MCOLN1, cv 81291 MTHFR, cv 81302 MECP2, fgs 81304 MECP2, dup_del 81324 PMP22, dup_del 81325 PMP22, fgs 1, V14 81326 PMP22, kfv 81330 SMPD1, cv 81331 SNRPN_UBE3A, ma 81350 UGT1A1, cv 81400 ABCC8, F1388del 81400 ACADM, K304E 81400 AGTR1, 1166A>C 81400 BCKDHA, Y438n 81400 CCR5, del 81400 CLRN1, N48K 81400 DPYD, IVS14+1G>A 81400 FGFR1, P252R 81400 FGFR3, P250R 81400 FKTN, retro ins v 81400 GNE, M712T 81400 HPA-6 81400 HPA-9 81400 IVD, A282V 81400 LCT, 13910 C>T 81400 NEB, exon 55 81400 PCDH15, R245X 81400 SHOC2, S2G 81400 SLCO1B1, V174A 81400 SMN1, exon 7 del 81400 SRY, fgs 81400 TOR1A, var 81401 ABCC8, cv 81401 ACADM, cv 81401 ADRB2, cv 81401 AFF2, detect abn 81401 APOB, cv 81401 APOE, cv 81401 AR, alleles 81401 ATN1 81401 ATXN1 81401 ATXN10 81401 ATXN2 81401 ATXN3 81401 ATXN7 81401 ATXN8OS 81401 CACNA1A 2, V14 81401 CBS, cv 81401 CFH_ARMS2, cv 81401 CNBP 81401 CSTB 81401 CYP3A4, cv 81401 CYP3A5, cv 81401 -

Flavin Adenine Dinucleotide Status and the Effects of High-Dose Riboflavin Treatment in Short-Chain Acyl-Coa Dehydrogenase Deficiency
0031-3998/10/6703-0304 Vol. 67, No. 3, 2010 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Flavin Adenine Dinucleotide Status and the Effects of High-Dose Riboflavin Treatment in Short-Chain Acyl-CoA Dehydrogenase Deficiency BIANCA T. VAN MALDEGEM, MARINUS DURAN, RONALD J. A. WANDERS, HANS R. WATERHAM, AND FRITS A. WIJBURG Department of Pediatrics [B.T.M, F.A.W.] and Laboratory Genetic Metabolic Diseases [M.D., R.J.A.W., H.R.W.], University of Amsterdam, 1105 AZ Amsterdam, the Netherlands ABSTRACT: Short-chain acyl-CoA dehydrogenase deficiency most US states (15). In addition, potential treatment options (SCADD) is an inborn error, biochemically characterized by in- for SCADD have never been systematically studied. creased plasma butyrylcarnitine (C4-C) concentration and increased SCADD is caused by decreased activity of the first enzyme ethylmalonic acid (EMA) excretion and caused by rare mutations of the short-chain fatty acid -oxidation spiral, which cata- and/or common gene variants in the SCAD encoding gene. Although lyzes the dehydrogenation of butyryl-CoA (C4-CoA). When its clinical relevance is not clear, SCADD is included in most US SCAD activity is impaired, C4-CoA will accumulate and is newborn screening programs. Riboflavin, the precursor of flavin subsequently converted into different metabolites including 1) adenine dinucleotide (FAD, cofactor), might be effective for treating the corresponding carnitine-ester, i.e. butyrylcarnitine (C4-C); SCADD. We assessed the FAD status and evaluated the effects of riboflavin treatment in a prospective open-label cohort study involv- 2) the corresponding glycine ester (butyrylglycine); 3) bu- ing 16 patients with SCADD, subdivided into mutation/mutation tyrate; and 4) ethylmalonic acid (EMA). -

1 Supplemental Information Development of an in Vitro Human
Supplemental Information Development of an In Vitro Human Liver System for Interrogating Non-Alcoholic Steatohepatitis Ryan Feaver1, Banumathi K. Cole1, Mark J. Lawson1, Stephen A. Hoang1, Svetlana Marukian1, Robert A. Figler1, Arun J. Sanyal2, Brian R. Wamhoff*1, Ajit Dash1 1HemoShear Therapeutics LLC, Charlottesville, VA 2Virginia Commonwealth University School of Medicine Richmond, VA *Corresponding Author: Brian R. Wamhoff, 501 Locust Ave., Charlottesville, VA 22902 [email protected] 434-872-0196 1 Supplemental Figures and Tables: log2 Fold Change Values ● ● ● ● ● ● ● ● 5 ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ●● ● ● ● ● ● ● ● ● ● ● ● ●● ● ● ● ● ● ● ● ● ● ● ● ● ● ●● ● ● ● ●● ● ● ● ● ● ● ●● ● ● ● ●●●● ●● ● ● ● ● ●●● ● ● ● ● ●● ●●●● ●●●●●● ● ● ● ● ● ●● ● ● ● ● ●● ●●●●●●●●●●●●●●●● ● ● ● ● ● ●●● ●●●●●●●●●●●●●● ● ● ● ●●●●●●●●●●●●●●●●● ● ●● ● ● ●●● ●● ●●●●●●●● ●●●●●●●●●●● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ●● ● ● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ●● ●●● ● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ● ● ● ● ●●●● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ●●● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ●● ● ● ● ● ●● ●● ● ●●● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ●●●●●● ● ● ●● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ●●● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ● ● ● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ● ●● ●●●●●●● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ●●● ●●●● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● ● ● ●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●●● -

Development of a Targeted Second-Tier Genetic Test For
Development of a Targeted Second-Tier Genetic Test for Conditions Examined during Newborn Screening 1Lonna Mollison, 1Chelsea Gustafson, 1Edgar A Rivera-Munoz, 1Alicia Brandt, 1Daniela DeCristo, 1Falecia Metcalf, 2Cynthia Powell, 1Jonathan Berg 1Department of Genetics, 2Department of Pediatrics and Genetics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina Introduction Figure 1. Library HEAT-Seq and SureSelectXT Comparison • Approximately four million children are born annually in the United States, preparation workflows for Agilent’s SureSelectXT most undergo state mandated newborn screening and Roche’s HEAT-Seq. A. B. C. • In 2006 the American College of Medical Genetics and Genomics (ACMG) • Time requirement for developed a recommended uniform screening panel (RUSP) to minimize SureSelectXT was about 32 hours, and variability between states for HEAT-Seq about 8 • Most RUSP conditions are detected by tandem mass spectrometry hours. • Recommended DNA • Through the use of genetic sequencing it is possible to detect the underlying input amount for genetic cause of RUSP conditions SureSelectXT was • Here we propose a step-wise approach to enhance traditional newborn about 3000ng, and for HEAT-Seq was about D. screening and integrate genetic screening into population health using cost- 250ng. effective, targeted sequencing to examine current RUSP conditions • Cost per sample for Figure 4. (A-C) The percent of bases at varying read depths for SureSelectXT HEAT-Seq and SureSelect when normalized to 5M reads (A), 50M (including all human reads (B) and 200M reads (C) for each of the 8 samples. (D) The exon baits) was about percent of targeted bases in all samples sequenced at 30 fold Core Condition Associated Gene(s) ACMG Code $333, and for HEAT- coverage were estimated normalizing the data to various values Maple Syrup Urine Disease BCKDHA, BCKDHB, DBT MSUD Seq (including MIPs) of sequenced reads. -

Details About Three Fatty Acid Oxidation Pathways Occurring in Man
Supplement information Details about three fatty acid oxidation pathways occurring in man Alpha oxidation Definition: Oxidation of the alpha carbon of the fatty acid, chain shortened by 1 carbon atom. Localization: Peroxisomes1 Substrates: Phytanic acid, 3-methyl fatty acids and their alcohol and aldehyde derivatives, metabolites of farnesol, geranylgeraniol, and dolichols2, 3. Steps in the pathway: Activation requires ATP and CoA. Hydroxylation requires iron, ascorbate and alpha-keto-glutarate as cofactors and secondary substrates. Lysis requires thymine pyrophosphate and magnesium ions. Dehydrogenation requires NADP. End products are transported into mitochondria for further oxidation. Enzymes and genes involved: Very long-chain acyl-CoA synthetase (E.C. 6.2.1.-) (SLC27A2, GeneID: 11001)4, phytanoyl-CoA dioxygenase (E.C. 1.14.11.18, PHYH, GeneID: 5264), 2-hydrosyphytanoyl-coA lyase (E.C. 4.1.-.-, HACL1, GeneID: 26061), and aldehyde dehydrogenase (E.C. 1.2.1.3, ALDH3A2, GeneID: 224). Disorders associated: Zellweger syndrome including RCDP type 1, where PTS2 receptor is defective and PHYH is unable to enter peroxisomes, and Refsum’s disease. Special features/ purpose: At the sub cellular level, the activation step can occur in the mitochondrion, endoplasmic reticulum, and peroxisome. Formic acid is the main byproduct of this pathway as opposed to carbon dioxide. Phytanic acid usually undergoes alpha oxidation; however, under conditions of enzyme deficiency, it undergoes omega oxidation and 3- methyladipic acid is produced as the end product5. Omega oxidation Definition: Oxidation of omega carbon of the fatty acid for generation of mono- and di- carboxylic acids. No chain shortening occurs. Localization: Fatty acid shuttles between cytosol and microsomes before entering the peroxisomes6. -

Elucidating the Mitochondrial Architecture of Branched-Chain Amino Acid Metabolism Enzymes: Implications for Treatment
ELUCIDATING THE MITOCHONDRIAL ARCHITECTURE OF BRANCHED-CHAIN AMINO ACID METABOLISM ENZYMES: IMPLICATIONS FOR TREATMENT by Stephen Paul McCalley BS Biology, University of Pittsburgh, 2013 Submitted to the Graduate Faculty of the Department of Human Genetics in the Graduate School of Public Health in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2018 UNIVERSITY OF PITTSBURGH GRADUATE SCHOOL OF PUBLIC HEALTH This dissertation was presented by Stephen Paul McCalley It was defended on February 23, 2018 and approved by Eric S Goetzman, PhD, Associate Professor, Human Genetics Graduate School of Public Health, University of Pittsburgh Candace Kammerer, PhD, Associate Professor, Human Genetics Graduate School of Public Health, University of Pittsburgh Zsolt Urban, PhD, Associate Professor, Human Genetics Graduate School of Public Health, University of Pittsburgh Dissertation Advisor: Jerry Vockley, MD, PhD, Professor, Human Genetics Graduate School of Public Health, University of Pittsburgh Copyright © by Stephen Paul McCalley 2018 Jerry Vockley, MD, PhD ELUCIDATING THE MITOCHONDRIAL ARCHITECTURE OF BRANCHED- CHAIN AMINO ACID METABOLISM ENZYMES: IMPLICATIONS FOR TREATMENT Stephen Paul McCalley, PhD University of Pittsburgh, 2018 ABSTRACT Branched chain amino acid (BCAA) metabolism occurs within the mitochondrial matrix and is comprised of 17 enzymes, some shared, organized into three pathways for the catabolism of leucine, isoleucine, and valine (LEU, ILE, and VAL respectively). However, the physical relationships of the various enzymes in the pathways are unknown. Diseases such as isovaleric academia, propionic and methylmalonic acidemias, and maple syrup urine disease are among the disorders caused by genetic deficiencies of BCAA metabolism. I examined the BCAA pathways and their physical interactions in vitro through proteomics analysis and in situ using stimulated emission depletion microscopy. -
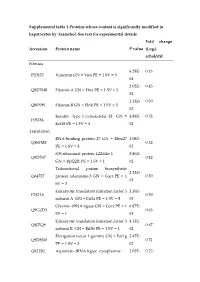
Supplemental Table 1 Proteins Whose Content Is Significantly Modified in Hepatocytes by Aramchol
Supplemental table 1 Proteins whose content is significantly modified in hepatocytes by Aramchol. See text for experimental details Fold change Accession Protein name P value (Log2) achol/ctrl Fibrosis 6.29E- 0.13 P20152 Vimentin GN = Vim PE = 1 SV = 3 04 2.02E- 0.45 Q8BTM8 Filamin-A GN = Flna PE = 1 SV = 5 02 1.13E- 0.50 Q80X90 Filamin-B GN = Flnb PE = 1 SV = 3 02 Keratin type I cytoskeletal 18 GN = 4.89E- 0.78 P05784 Krt18 PE = 1 SV = 5 02 Translation RNA-binding protein 27 GN = Rbm27 1.08E- Q5SFM8 0.32 PE = 1 SV = 3 02 60S ribosomal protein L22-like 1 3.86E- Q9D7S7 0.42 GN = Rpl22l1 PE = 1 SV = 1 02 Trifunctional purine biosynthetic 2.33E- Q64737 protein adenosine-3 GN = Gart PE = 1 0.50 03 SV = 3 Eukaryotic translation initiation factor 3 3.10E- P23116 0.59 subunit A GN = Eif3a PE = 1 SV = 5 03 Glycine--tRNA ligase GN = Gars PE = 1 6.87E- Q9CZD3 0.63 SV = 1 03 Eukaryotic translation initiation factor 3 4.13E- Q8JZQ9 0.67 subunit B GN = Eif3b PE = 1 SV = 1 02 Elongation factor 1-gamma GN = Eef1g 2.47E- Q9D8N0 0.71 PE = 1 SV = 3 02 Q922B2 Aspartate--tRNA ligase cytoplasmic 2.02E- 0.72 GN = Dars PE = 1 SV = 2 02 Arginine--tRNA ligase cytoplasmic 1.47E- Q9D0I9 0.72 GN = Rars PE = 1 SV = 2 02 RNA-binding protein 25 GN = Rbm25 1.28E- B2RY56 1.92 PE = 1 SV = 2 02 Eukaryotic translation initiation factor 4 7.92E- Q80XI3 3.90 gamma 3 GN = Eif4g3 PE = 1 SV = 2 03 Lipid metabolism Prostaglandin reductase 1 GN = Ptgr1 4.55E- Q91YR9 0.42 PE = 1 SV = 2 02 3.09E- Q9DBG5 Perilipin-3 GN = Plin3 PE = 1 SV = 1 0.51 02 Acyl-CoA dehydrogenase family 3.98E- -

Dynamic Modelling of an ACADS Genotype in Fatty Acid Oxidation – Application of Cellular Models for the Analysis of Common Genetic Variants
RESEARCH ARTICLE Dynamic modelling of an ACADS genotype in fatty acid oxidation ± Application of cellular models for the analysis of common genetic variants Kerstin Matejka1,2,3,4,5☯, Ferdinand StuÈ ckler6☯, Michael Salomon7, Regina Ensenauer8,9,10, Eva Reischl11,12, Lena Hoerburger13, Harald Grallert3,4,5,11,12, Gabi KastenmuÈ ller14, Annette Peters3,12,15, Hannelore Daniel2,16, Jan Krumsiek6,17, Fabian J. Theis6,18*, 1,2,3,4,5,19 1,2,3,4,5,13,20,21 a1111111111 Hans Hauner , Helmut LaumenID * a1111111111 a1111111111 1 Chair of Nutritional Medicine, Else KroÈner-Fresenius-Center for Nutritional Medicine, TUM School of Life Sciences Weihenstephan, Technische UniversitaÈt MuÈnchen, Freising-Weihenstephan, Germany, 2 ZIEL- a1111111111 Research Center for Nutrition and Food Sciences, TUM School of Life Sciences Weihenstephan, Technische a1111111111 UniversitaÈt MuÈnchen, Freising-Weihenstephan, Germany, 3 German Center for Diabetes Research (DZD), Neuherberg, Germany, 4 Clinical Cooperation Group Nutrigenomics and Type 2 Diabetes, Helmholtz Zentrum MuÈnchen, Neuherberg, Germany, 5 Clinical Cooperation Group Nutrigenomics and Type 2 Diabetes, Technische UniversitaÈt MuÈnchen, Freising-Weihenstephan, Germany, 6 Institute of Computational Biology, Helmholtz Zentrum MuÈnchen, Neuherberg, Germany, 7 SIRION Biotech GmbH, Martinsried, Germany, 8 Research Center, Dr. von Hauner Children's Hospital, Ludwig-Maximilians-UniversitaÈt MuÈnchen, OPEN ACCESS MuÈnchen, Germany, 9 Experimental Pediatrics and Metabolism, Department of General Pediatrics, Citation: -

Lipid Myopathies
Journal of Clinical Medicine Review Lipid Myopathies Elena Maria Pennisi 1,*, Matteo Garibaldi 2 and Giovanni Antonini 2 1 Unit of Neuromuscular Disorders, Neurology, San Filippo Neri Hospital, 00135 Rome, Italy 2 Unit of Neuromuscular Diseases, Department of Neurology, Mental Health and Sensory Organs (NESMOS), SAPIENZA University of Rome, Sant’ Andrea Hospital, 00189 Rome, Italy; [email protected] (M.G.); [email protected] (G.A.) * Correspondence: [email protected] or [email protected]; Tel.: +39-(06)-3306-2374 Received: 28 October 2018; Accepted: 17 November 2018; Published: 23 November 2018 Abstract: Disorders of lipid metabolism affect several tissues, including skeletal and cardiac muscle tissues. Lipid myopathies (LM) are rare multi-systemic diseases, which most often are due to genetic defects. Clinically, LM can have acute or chronic clinical presentation. Disease onset can occur in all ages, from early stages of life to late-adult onset, showing with a wide spectrum of clinical symptoms. Muscular involvement can be fluctuant or stable and can manifest as fatigue, exercise intolerance and muscular weakness. Muscular atrophy is rarely present. Acute muscular exacerbations, resulting in rhabdomyolysis crisis are triggered by several factors. Several classifications of lipid myopathies have been proposed, based on clinical involvement, biochemical defect or histopathological findings. Herein, we propose a full revision of all the main clinical entities of lipid metabolism disorders with a muscle involvement, also including some those disorders of fatty acid oxidation (FAO) with muscular symptoms not included among previous lipid myopathies classifications. Keywords: lipid myopathies; lipid storage disease; muscle lipidosis; lipid metabolism disorders; beta-oxidation defects; FAO defect; metabolic myopathies 1.