CMA Banding Patterns of Chromosomes in Major Citrus Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -
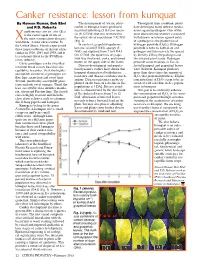
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

PRUNING GUIDELINES Tools: Loppers, Saw, Clippers 10% Bleach and Water Solution
Varieties - choose one that you will want to eat often, as you will have them much of the year (Four Winds Citrus Variety Chart link in your Resources list) - certain citrus mature earlier than others (see early ripening handout) - Unique varieties: - Blood orange: red flesh is antioxidant rich. Often sweeter than other oranges - Yuzu: very little, but very flavorful juice used by chefs. Believed to be a cross between a sour mandarin and ichang papeda - Keiffir lime: regular lime with bumpy skin. Attractive tree with segmented leaves that are extremely fragrant and prized by chefs. - Buddhas hand: not much juice, but very fragrant pith and rind. Odd shaped and often used as an ornamental - Australian finger lime: oblong green lime with many small lime-flavored orbs inside. Called "citrus caviar" Standard varieties of citrus trees often grow to a height of 20 to 30 feet and the canopy -- or width of a tree -- can spread to 18 to 30 feet depending on the variety. Dwarf citrus trees are significantly shorter and narrower, which provides greater flexibility in planting location. Most varieties top out at 8 feet in height with a proportionally smaller canopy. Despite the differences in height and width, regular, semi-dwarf and dwarf citrus varieties produce the same size fruit. -First off DO NOT PRUNE! Those damaged leaves can actually provide protection for the plant until the air warms up. The plant needs to rally and recover. Pruning might just put it over the edge. Sometimes the plants must remain with that ‘raggedy’ appearance until as late as June and in some cases a full year. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -

Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives
plants Article Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives Clémentine Baccati 1, Marc Gibernau 1, Mathieu Paoli 1 , Patrick Ollitrault 2,3 ,Félix Tomi 1,* and François Luro 2 1 Laboratoire Sciences Pour l’Environnement, Equipe Chimie et Biomasse, Université de Corse—CNRS, UMR 6134 SPE, Route des Sanguinaires, 20000 Ajaccio, France; [email protected] (C.B.); [email protected] (M.G.); [email protected] (M.P.) 2 UMR AGAP Institut, Université Montpellier, CIRAD, INRAE, Institut Agro, 20230 San Giuliano, France; [email protected] (P.O.); [email protected] (F.L.) 3 CIRAD, UMR AGAP, 20230 San Giuliano, France * Correspondence: [email protected]; Tel.: +33-495-52-4122 Abstract: The Papeda Citrus subgenus includes several species belonging to two genetically distinct groups, containing mostly little-exploited wild forms of citrus. However, little is known about the potentially large and novel aromatic diversity contained in these wild citruses. In this study, we characterized and compared the essential oils obtained from peels and leaves from representatives of both Papeda groups, and three related hybrids. Using a combination of GC, GC-MS, and 13C-NMR spectrometry, we identified a total of 60 compounds in peel oils (PO), and 76 compounds in leaf oils (LO). Limonene was the major component in almost all citrus PO, except for C. micrantha and C. hystrix, where β-pinene dominated (around 35%). LO composition was more variable, with different Citation: Baccati, C.; Gibernau, M.; major compounds among almost all samples, except for two citrus pairs: C. -

Citrus Rootstocks: Their Characters and Reactions
CITRUS ROOTSTOCKS: THEIR CHARACTERS AND REACTIONS (an unpublished manuscript) ca. 1986 By W. P. BITTERS (1915 – 2006) Editor, digital version: Marty Nemeth, Reference Librarian, UC Riverside Science Library, retired Subject matter experts, digital version: Dr. Tracy Kahn, Curator, UC Citrus Variety Collection Dr. Robert Krueger, Curator, USDA-ARS National Clonal Germplasm Repository for Citrus & Dates Toni Siebert, Assistant Curator, UC Citrus Variety Collection ca. 1955 ca. 1970 IN MEMORIUM Willard P. Bitters Professor of Horticulture, Emeritus Riverside 1915-2006 Born in Eau Claire, Wisconsin, in June, 1915, Dr. Willard “Bill” Bitters earned his bachelor’s degree in biology from St. Norbert College and his master’s degree and Ph.D. from the University of Wisconsin. After earning his doctorate, he first worked as the superintendent of the Valley Research Farm of the University of Arizona in Yuma, and joined the Citrus Experiment Station, in Riverside in 1946 as a Horticulturist. In 1961, Dr. Bitters became a Professor in the newly established University of California-Riverside. His initial assignment was to work on horticultural aspects of tristeza, a serious vector-transmitted virus disease which threatened to destroy California citrus orchards. Tristeza was already in California and spreading in 1946. At that time most citrus trees in California were grafted on a rootstock that was known to be susceptible to tristeza. Dr. Bill Bitters was responsible for screening of over 500 cultivars to determine which rootstock-scion combinations were resistant to this disease and yet possessed suitable horticultural characteristics. Of the 500 screened, most were susceptible, but several successful ones were selected and released to the industry. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

Macrophylla Is Highly Resistant to Phytophthora and Tolerant of Exocortis
CITRUS ROOTSTOCKS FROM THE PAPEDA GROUP by W. P. Bitters, C, D. McCarty and D, A. Cole* Many citrus and near-citrus relatives have been tried as rootstocks. Of interest are some of the species and hybrids of the subgenus Papeda, the fruits of which differ from citrus mainly in that they are unedible because the pulp vesicles contain an acrid oil that gives the fruit a disagreeable flavor. Among the most widely used as rootstocks from the Papeda group are the naturally occurring hybrids Yuzu and Citrus macrophylla or Alemow. =, C. macrophylla has gained wide acceptance in California and Arizona as a rootstock for lemons. Seedlings of macrophylla are very vigorous and require a minimum of nursery care. Macrophylla buds well with all scion varieties of citrus, but its susceptibility to tristeza and cachexia rule it out for use in most areas except with lemons and limes. Macrophylla is highly resistant to Phytophthora and tolerant of exocortis. It does well on all soil types from sandy to heavy clay loams and has a high degree of resistance to calcareous and saline soils. *W. P. Bitters and D. A. Cole are members of the Department of Plant Science, CRC, UCR. C.D. McCarty is a member of the Agricultural Extension Service, UCR. -2- It shows perhaps the highest boron tolerance of all citrus rootstocks, and is also capable of absorbing micronutrients in slightly greater quantities than other citrus. Hence, trees on macrophylla are deep green in color and seldom show leaf patterns that denote micronutrient deficiencies. Young lemon trees on macrophylla are vigorous and precocious in bearing. -

Genomic Analyses of Primitive, Wild and Cultivated Citrus Provide Insights Into Asexual Reproduction
ARTICLES OPEN Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction Xia Wang1,7, Yuantao Xu1,7, Siqi Zhang1,7, Li Cao2,7, Yue Huang1, Junfeng Cheng3, Guizhi Wu1, Shilin Tian4, Chunli Chen5, Yan Liu3, Huiwen Yu1, Xiaoming Yang1, Hong Lan1, Nan Wang1, Lun Wang1, Jidi Xu1, Xiaolin Jiang1, Zongzhou Xie1, Meilian Tan1, Robert M Larkin1, Ling-Ling Chen3, Bin-Guang Ma3, Yijun Ruan5,6, Xiuxin Deng1 & Qiang Xu1 The emergence of apomixis—the transition from sexual to asexual reproduction—is a prominent feature of modern citrus. Here we de novo sequenced and comprehensively studied the genomes of four representative citrus species. Additionally, we sequenced 100 accessions of primitive, wild and cultivated citrus. Comparative population analysis suggested that genomic regions harboring energy- and reproduction-associated genes are probably under selection in cultivated citrus. We also narrowed the genetic locus responsible for citrus polyembryony, a form of apomixis, to an 80-kb region containing 11 candidate genes. One of these, CitRWP, is expressed at higher levels in ovules of polyembryonic cultivars. We found a miniature inverted-repeat transposable element insertion in the promoter region of CitRWP that cosegregated with polyembryony. This study provides new insights into citrus apomixis and constitutes a promising resource for the mining of agriculturally important genes. Asexual reproduction is a remarkable feature of perennial fruit crops important trait for breeding purposes. On the one hand, polyembryony that facilitates the faithful propagation of commercially valuable is widely employed in citrus nurseries and propagation programs to individuals by avoiding the uncertainty associated with the sexual generate large numbers of uniform rootstocks from seeds. -

Descriptions of New Varieties Recently Distributed from the Citrus Clonal Protection Program
Descriptions of new varieties recently distributed from the Citrus Clonal Protection Program Toni Siebert, Robert Krueger, Tracy Kahn, John Bash and Georgios Vidalakis he Citrus Clonal Protection Program (CCPP) Protected Foundation Block Budwood is operated through the Department of Plant “Protected Foundation Block Budwood” is budwood TPathology and Microbiology at University of provided from CDFA registered CCPP citrus trees from the California (UC) Riverside and is funded in large part LREC screenhouses and is available from the University of by The California Citrus Research Board (CRB). The California in accordance with the CDFA regulations for cit- CCPP processes citrus propagative material in two rus registration and certification. Protected Foundation Block phases. First, during the quarantine phase, citrus bud- Budwood is produced by trees grown in pots and in ground wood of potentially important commercial varieties is under protective screen and is intended for individual nurseries introduced from any citricultural area, germplasm or or growers to produce their own registered budwood source breeding program of the world under the authority of trees or for the production of nursery increase blocks from a permit which is issued to CCPP by the United States which additional budwood may be harvested in accordance Department of Agriculture (USDA) Animal and Plant with CDFA (or other appropriate) regulations and used for Health Inspection Service in cooperation with the Cali- the production of certified nursery stock. A signed “Waiver fornia Department of Food and Agriculture (CDFA). and Release” form must accompany all orders for Protected While in quarantine at the Rubidoux Facility in River- Foundation Block Budwood. -

Citrus Phylogeny and Genetic Origin of Important Species As Investigated by Molecular Markers
Theor Appl Genet (2000) 100:1155–1166 © Springer-Verlag 2000 ORIGINAL PAPER E. Nicolosi · Z.N. Deng · A. Gentile · S. La Malfa G. Continella · E. Tribulato Citrus phylogeny and genetic origin of important species as investigated by molecular markers Received: 5 October 1999 / Accepted: 3 November 1999 Abstract Citrus phylogeny was investigated using for Rangpur lime and Rough lemon. For Mexican lime RAPD, SCAR and cpDNA markers. The genotypes ana- our molecular data indicated C. micrantha to be the fe- lyzed included 36 accessions belonging to Citrus to- male parent and C. medica as the male one. gether with 1 accession from each of the related genera Poncirus, Fortunella, Microcitrus and Eremocitrus. Key words Citrus · RAPD · SCAR · cpDNA · Phylogenetic analysis with 262 RAPDs and 14 SCARs Phylogeny · Origin indicated that Fortunella is phylogenetically close to Citrus while the other three related genera are distant from Citrus and from each other. Within Citrus, the sep- Introduction aration into two subgenera, Citrus and Papeda, desig- nated by Swingle, was clearly observed except for C. Different hypotheses have been formulated on the origin celebica and C. indica. Almost all the accessions be- of Citrus. In general, Citrus is believed to have originat- longing to subgenus Citrus fell into three clusters, each ed in the tropical and subtropical regions of Southeast including 1 genotype that was considered to be a true Asia and then spread to other continents (Webber 1967; species. Different phylogenetic relationships were re- Calabrese 1992). Citrus taxonomy and phylogeny, how- vealed with cpDNA data. Citrus genotypes were sepa- ever, are very complicated, controversial and confusing, rated into subgenera Archicitrus and Metacitrus, as pro- mainly due to sexual compatibility between Citrus and posed by Tanaka, while the division of subgenera Citrus related genera, the high frequency of bud mutations and and Papeda disappeared. -

Reading: Classification of Citrus
Source: A.H. Krezdorn, Department of Fruit Crops, University of Florida Reading 32 Classifi cation of Citrus The genus Citrus contains many kinds or types that differ as to their fruits, fl owers, leaves, and twigs. The arranging of the kinds of Citrus into groups (as indicated by features such as looseness of peel, size, shape, and color) is termed classifi cation. The naming of these groups with valid names is termed nomenclature. There are defi nite international rules of nomenclature but not of classifi cation. Several per- sons have placed the various kinds of Citrus into groups (classifi ed them) and given them valid names. The classifi cation one accepts is one of personal choice, based on utility, common usage and natural relationships. Currently, there are 2 outstanding systems of classifi cation for Citrus. They are those of Wal- ter T. Swingle, a USDA scientist who did much of his work in Florida, and Tyosaburo Tanaka of Japan. Swingle’s system is relatively simple, containing 16 species. He is commonly referred to as a “lumper” because he lumps a large number of kinds into a relatively small number of groups. Tanaka’s system initially included 145 species and he is known as a “splitter” because he has split the genus Citrus into many small groups. He has continued to add to this list. From the standpoint of the grower, most horticulturists and other plant scientists, Swingle’s system appears the most useable. Tanaka’s system has Some features that are more reasonable than some of Swingle’s; however, it is not in total a very convenient or botanically sound system.