Neuroimaging Evidence Implicating Cerebellum in Support of Sensory Cognitive Processes Associated with Thirst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

Introduction
Cambridge University Press 978-1-316-64693-9 — The Brain and Behavior 4th Edition Excerpt More Information Chapter1 Introduction Introduction Theneuraxisinthehumanrunsasanimaginary straight line through the center of the spinal cord Human behavior is a direct reflection of the anatomy and brainstem (Figure 1.1). At the level of the junc- and physiology of the central nervous system. The goal tion of the midbrain and diencephalon, however, the of the behavioral neuroscientist is to uncover the neu- neuraxis changes orientation and extends from the roanatomical substrates of behavior. Complex mental occipital pole to the frontal pole (Figure 1.1). processes are represented in the brain by their elemen- The neuraxis located above the midbrain is the neur- tary components. Elaborate mental functions consist of axis of the cerebrum and is sometimes called the subfunctions and are constructed from both serial and horizontal neuraxis. A cross-section taken perpendi- parallel interconnections of several brain regions. cular to the horizontal neuraxis is called a coronal An introduction to the nervous system covers general (frontal) section. terminology and the ventricular system. With regard to the neuraxis of the spinal cord and brainstem: Major Subdivisions • Dorsal (posterior) means toward the back. The nervous system is divided anatomically into the • Ventral (anterior) means toward the abdomen. central nervous system (CNS) and the peripheral ner- • Rostral means toward the nose. vous system (PNS). • Caudal means toward the tail. • The CNS is made up of the brain and spinal cord. • The sagittal (midsagittal) plane is the vertical • The PNS consists of the cranial nerves and spinal plane that passes through the neuraxis. -

Occurrence of Long-Term Depression in the Cerebellar Flocculus During Adaptation of Optokinetic Response Takuma Inoshita, Tomoo Hirano*
SHORT REPORT Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response Takuma Inoshita, Tomoo Hirano* Department of Biophysics, Graduate School of Science, Kyoto University, Sakyo-ku, Japan Abstract Long-term depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a main cellular mechanism for motor learning. However, the necessity of LTD for motor learning was challenged by demonstration of normal motor learning in the LTD-defective animals. Here, we addressed possible involvement of LTD in motor learning by examining whether LTD occurs during motor learning in the wild-type mice. As a model of motor learning, adaptation of optokinetic response (OKR) was used. OKR is a type of reflex eye movement to suppress blur of visual image during animal motion. OKR shows adaptive change during continuous optokinetic stimulation, which is regulated by the cerebellar flocculus. After OKR adaptation, amplitudes of quantal excitatory postsynaptic currents at PF-PC synapses were decreased, and induction of LTD was suppressed in the flocculus. These results suggest that LTD occurs at PF-PC synapses during OKR adaptation. DOI: https://doi.org/10.7554/eLife.36209.001 Introduction The cerebellum plays a critical role in motor learning, and a type of synaptic plasticity long-term *For correspondence: depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a primary [email protected]. cellular mechanism for motor learning (Ito, 1989; Hirano, 2013). However, the hypothesis that LTD ac.jp is indispensable for motor learning was challenged by demonstration of normal motor learning in Competing interests: The rats in which LTD was suppressed pharmacologically or in the LTD-deficient transgenic mice authors declare that no (Welsh et al., 2005; Schonewille et al., 2011). -

Nucleus Dorsalis Superficialis (Lateralis Dorsalis) of the Thalamus and the Limbic System in Man
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.7.765 on 1 July 1974. Downloaded from Joutrnal of Neur)ology, Neurosurgery, and Psychiatry, 1974, 37, 765-789 Nucleus dorsalis superficialis (lateralis dorsalis) of the thalamus and the limbic system in man J. M. VAN BUREN AND R. C. BORKE Fr-om the Surgical Neurology Branch, National Institute of Neurological Diseases and Str-oke, National Institutes of Health, Bethesda, Maryland, U.S.A. SYNOPSIS Although the earlier supposition was that the n. dorsalis superficialis (n. lateralis dorsalis) of the thalamus projected to the parietal region, more recent evidence has linked it to the posterior cingulate gyrus and possibly adjacent regions near the splenium of the corpus callosum. An afferent supply from lower levels was in more doubt, although some report had been made of cell and fibre degeneration in the n. dorsalis superficialis after extensive temporal resections and section of the fornix in lower primates. The five human hemispheres of the present study all had lesions of long duration below the level of the splenium of the corpus callosum in the posteromedial temporal region. All showed marked degeneration in the fornix and n. dorsalis superficialis. In favourably Protected by copyright. stained cases, gliotic fascicles could be followed from the descending column of the fornix to the n. dorsalis superficialis via the region lateral to the stria medullaris thalami. The cell loss in the nucleus thus appeared to be an instance of anterograde transynaptic degeneration. These cases provided an interesting instance in which human infarctions provided natural lesions that would have been hard to duplicate in experimental animals. -

Neuroimaging Evidence Implicating Cerebellum in the Experience of Hypercapnia and Hunger for Air
Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air Lawrence M. Parsons*†, Gary Egan‡, Mario Liotti*, Stephen Brannan*, Derek Denton‡, Robert Shade§, Rachael Robillard¶, Lisa Madden§, Bart Abplanalp¶, and Peter T. Fox* *Research Imaging Center, University of Texas Health Science Center, San Antonio, TX 78284; ‡Howard Florey Institute of Experimental Physiology and Medicine, University of Melbourne, Parkville, Victoria 3052, Australia; §Southwest Foundation for Biomedical Research, P. O. Box 760549, San Antonio, TX 78245-0549; and ¶Departments of Psychology and Educational Psychology, University of Texas, Austin, TX 78712 Contributed by Derek Denton, December 20, 2000 Recent neuroimaging and neurological data implicate cerebellum areas (20–23). Anatomical tracer labeling data in rat (24) in nonmotor sensory, cognitive, vegetative, and affective func- indicate that a number of cerebellar areas send and͞or receive tions. The present study assessed cerebellar responses when the projections from the VRG, which contains the structures nec- urge to breathe is stimulated by inhaled CO2. Ventilation changes essary for respiratory rhythm generation (25–30). The cerebellar follow arterial blood partial pressure CO2 changes sensed by the areas connected to the VRG are quadrangular (VI), central medullary ventral respiratory group (VRG) and hypothalamus, (III), lingula (I, II), and inferior semilunar (Crus II) lobules, as entraining changes in midbrain, pons, thalamus, limbic, paralimbic, well as fastigial nucleus, interposed nucleus, and dentate nuclei, and insular regions. Nearly all these areas are known to connect the output nuclei for the vermal, intermediate, and lateral anatomically with the cerebellum. Using positron emission tomog- regions of cerebellum. Moreover, cerebellum has known con- raphy, we measured regional brain blood flow during acute CO2- nectivity to the hypothalamus, thalamus, pons, and midbrain induced breathlessness in humans. -

…Going One Step Further
…going one step further C20 (1017868) 2 Latin A Encephalon Mesencephalon B Telencephalon 31 Lamina tecti B1 Lobus frontalis 32 Tegmentum mesencephali B2 Lobus temporalis 33 Crus cerebri C Diencephalon 34 Aqueductus mesencephali D Mesencephalon E Metencephalon Metencephalon E1 Cerebellum 35 Cerebellum F Myelencephalon a Vermis G Circulus arteriosus cerebri (Willisii) b Tonsilla c Flocculus Telencephalon d Arbor vitae 1 Lobus frontalis e Ventriculus quartus 2 Lobus parietalis 36 Pons 3 Lobus occipitalis f Pedunculus cerebellaris superior 4 Lobus temporalis g Pedunculus cerebellaris medius 5 Sulcus centralis h Pedunculus cerebellaris inferior 6 Gyrus precentralis 7 Gyrus postcentralis Myelencephalon 8 Bulbus olfactorius 37 Medulla oblongata 9 Commissura anterior 38 Oliva 10 Corpus callosum 39 Pyramis a Genu 40 N. cervicalis I. (C1) b Truncus ® c Splenium Nervi craniales d Rostrum I N. olfactorius 11 Septum pellucidum II N. opticus 12 Fornix III N. oculomotorius 13 Commissura posterior IV N. trochlearis 14 Insula V N. trigeminus 15 Capsula interna VI N. abducens 16 Ventriculus lateralis VII N. facialis e Cornu frontale VIII N. vestibulocochlearis f Pars centralis IX N. glossopharyngeus g Cornu occipitale X N. vagus h Cornu temporale XI N. accessorius 17 V. thalamostriata XII N. hypoglossus 18 Hippocampus Circulus arteriosus cerebri (Willisii) Diencephalon 1 A. cerebri anterior 19 Thalamus 2 A. communicans anterior 20 Sulcus hypothalamicus 3 A. carotis interna 21 Hypothalamus 4 A. cerebri media 22 Adhesio interthalamica 5 A. communicans posterior 23 Glandula pinealis 6 A. cerebri posterior 24 Corpus mammillare sinistrum 7 A. superior cerebelli 25 Hypophysis 8 A. basilaris 26 Ventriculus tertius 9 Aa. pontis 10 A. -

The Cerebellum: 3
41 The Cerebellum: 3. Anatomic-MR Correlation in the Coronal Plane Gary A. Press 1 Thin (5-mm) coronal high-field (1.5-T) MR images of four human brain specimens and James W. Murakami2 14 normal volunteers were correlated with myelin-stained microtomic sections of the Eric Courchesne2 specimen cerebella. The primary white-matter tracts innervating several hemispheric Marjorie Grafe3 (posterior quadrangular, superior, and inferior semilunar, gracile, biventer, tonsil) and John R. Hesselink1 vermian (declive, folium, tuber) lobules are oriented perpendicularly to the coronal plane of section and are shown well on proton-density-weighted (long TR/short TE) and T2- weighted (long TR/Iong TE) spin-echo images, which provide excellent contrast between gray and white matter. Several of the surface sulci and fissures of the cerebellar hemispheres (including the superior posterior, horizontal, secondary, and posterolateral fissures) also course perpendicular to the coronal plane and are depicted well on T1- weighted (short TR/short TE) and T2-weighted images, which maximize contrast be tween CSF and parenchyma. The opportunity for side-to-side comparison of the hemi spheres is a distinct advantage of the coronal view. Nevertheless, more obliquely oriented surfaces (preculminate, primary, inferior posterior, inferior anterior, and intra biventral fissures) and deep hemispheric structures (primary white-matter tracts to central, anterior quadrangular, and floccular lobules) may be obscured by volume averaging in the coronal plane; moreover, much of the finer anatomy of the vermis is depicted poorly. The constant surface and deep anatomy of the cerebellum revealed on coronal images in normal volunteers encourages detailed mapping. MR imaging in the coronal plane should be especially useful in identifying, localizing, and quantifying normal and abnormal morphologic differences between the cerebellar hemispheres. -

Is Composed from Spinal Cord and Brain
doc. MUDr. Adriana Boleková, PhD. MVDr. Natália Hvizdošová, PhD. CENTRAL NERVOUS SYSTEM – is composed from spinal cord and brain SPINAL CORD cranial border: foramen magnum, pyramidal decussation, exit of first pair of spinal nerves caudal border: level of L1 – L2 vertebrae medullary cone – filum terminale (S2) – cauda equina enlargements: cervical enlargement (C5 – Th1): origin of nerves for upper extremity – brachial plexus lumbosacral enlargement (L1 – S2): origin of nerves for lower extremity – lumbosacral plexus external features: anterior median fissure anterolateral sulcus – anterior roots of spinal nn. posterolateral sulcus – posterior roots of spinal nn. posterior median sulcus posterior intermediate sulcus internal features: White matter anterior funiculus (between anterior median fissure and anterolateral sulcus) lateral funiculus (between anterolateral and posterolateral sulci) posterior funiculus (between posterolateral sulcus and posterior median sulcus) fasciculus gracilis fasciculus cuneatus Gray matter anterior (ventral) horn – motor function: Rexed laminae I – VI lateral horn – serves to visceral function: Rexed lamina VII dorsal (posterior) horn – sensory information: Rexed laminae VIII – IX central grey matter – interneurons: around central canal Rexed lamina X Central canal cranially opens into IV. ventricle caudally expands into terminal ventricle vessels of spinal cord: Arteries: spinal brr. from surrounding arteries – anterior radicular aa., posterior radicular aa.: posterior spinal aa. (in posterolateral -
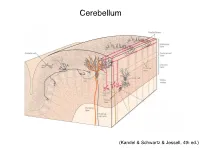
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

On the Function of the Floccular Complex of the Vertebrate Cerebellum: Implications in Paleoneuroanatomy
On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes Successfully defended on 18th November 2015 at FCT-UNL Campus, Portugal, before a juri presided over by: Doutor Paulo Alexandre Rodrigues Roque Legoinha and consisting of: Doutor Gabriel José Gonçalves Martins Doutor Rui Alexandre Ferreira Castanhinha I II Direitos de autor - Copyright Os direitos de autor deste documento pertencem a Sérgio Filipe Ferreira Cardoso, à FCT/UNL, à UNL e à UÉ. A Faculdade de Ciências e Tecnologia, a Universidade Nova de Lisboa e a Universidade de Évora têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. Two peer-reviewed abstracts, resulting from this study, were accepted for oral communications (Appendix II). Ferreira-Cardoso, S., Araújo, R., Castanhinha, R., Walsh, S., Martins, R.M.S., Martins, G.G. -

Computed Tomography of the Brain Stem with Intrathecal Metrizamide. Part I: the Normal Brain Stem
Computed Tomography of the Brain Stem with Intrathecal Metrizamide. Part I: The Normal Brain Stem Michel E. Mawad 1 Detailed anatomy of the brain stem and cervicomedullary junction can be accurately A. John Silver demonstrated with metrizamide computed tomographic cisternography. Specifically. Sadek K. Hilal surface anatomy is unusually well outlined. Nine distinct and easily recognizable levels S. Ramaiah Ganti of section are described: four levels in the medulla, three in the pons, and two in the mesencephalon. Surface features of the brain stem, fine details in the floor of the fourth ventricle, cranial nerves, and vascular structures are shown and discussed. Reliably accurate imaging of the brain stem and cervicomedullary junction has now become available using high-resolution computed tomographic (CT) scan ning following intrathecal admini stration of metrizamide [1 -6]. The demonstration of surface features of the brain stem such as the ventral fissure, ventrolateral su lcus, pyramids, and olivary protuberance has become commonplace; suc h details have not been routinely demonstrable in the past. Many authors [1, 2] have already emphasized the value of metrizamide CT cisternography and its superiority to both angiography and pneumoencephalog raphy. These latter procedures rely on subtle displacement of vessels or distor tion of the air-filled fourth ventricle and posterior fossa cisterns. Compared with air, metrizamide spreads much more readily in th e entire subarachnoid space without the problem of meniscus formation or " air lock. " CT permits the sepa ration of the various collections of contrast agent and avoids th e superimposition of features encountered in nontomographic contrast studies. Improved visualization of the details of the brain stem by metrizamide CT has allowed the detection of subtle morphologic changes in the brain stem and subarachnoid space not previously appreciated. -

The Cerebellum in Eye Movement Control
Eye (2015) 29, 191–195 & 2015 Macmillan Publishers Limited All rights reserved 0950-222X/15 www.nature.com/eye CAMBRIDGE OPHTHALMOLOGICAL SYMPOSIUM The cerebellum in VR Patel1 and DS Zee2 eye movement control: nystagmus, coordinate frames and disconjugacy This article has been corrected since Advance Online Publication and an erratum is also printed in this issue Abstract cerebellum in binocular control—both to create disconjugacy when it is necessary, and to In this review we discuss several aspects of prevent ocular misalignment when it is eye movement control in which the unnecessary and disruptive. We will also touch cerebellum is thought to have a key role, but on the implications of evolving into frontal-eyed have been relatively ignored. We will focus creatures, with the competing demands on the mechanisms underlying certain forms of binocular, foveal vs retinal, full-field of cerebellar nystagmus, as well as the stabilization of images. Furthermore, we contributions of the cerebellum to binocular suggest that the phylogenetically old vestibular alignment in healthy and diseased states. anlage persists in a rudimentary form within A contemporary review of our understanding our human brains and its vestiges can be provides a basis for directions of further 1Associate Professor of uncovered in neurological disease.1–4 These inquiry to address some of the uncertainties Ophthalmology, USC Eye issues bear on interpretation of pathological regarding the contributions of the cerebellum Institute, Keck School of nystagmus, which depends on the coordinate Medicine, University of to ocular motor control. system in which the nystagmus is couched Southern California, Eye (2015) 29, 191–195; doi:10.1038/eye.2014.271; Los Angeles, CA, USA (foveal: eye frame vs full-field retina: head published online 14 November 2014 frame), and also on the types of ocular 2Professor of Neurology, misalignment seen in cerebellar patients.