Managing Woody Bush Encroachment Impacting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Timeline of Natural History
Timeline of natural history This timeline of natural history summarizes significant geological and Life timeline Ice Ages biological events from the formation of the 0 — Primates Quater nary Flowers ←Earliest apes Earth to the arrival of modern humans. P Birds h Mammals – Plants Dinosaurs Times are listed in millions of years, or Karo o a n ← Andean Tetrapoda megaanni (Ma). -50 0 — e Arthropods Molluscs r ←Cambrian explosion o ← Cryoge nian Ediacara biota – z ←Earliest animals o ←Earliest plants i Multicellular -1000 — c Contents life ←Sexual reproduction Dating of the Geologic record – P r The earliest Solar System -1500 — o t Precambrian Supereon – e r Eukaryotes Hadean Eon o -2000 — z o Archean Eon i Huron ian – c Eoarchean Era ←Oxygen crisis Paleoarchean Era -2500 — ←Atmospheric oxygen Mesoarchean Era – Photosynthesis Neoarchean Era Pong ola Proterozoic Eon -3000 — A r Paleoproterozoic Era c – h Siderian Period e a Rhyacian Period -3500 — n ←Earliest oxygen Orosirian Period Single-celled – life Statherian Period -4000 — ←Earliest life Mesoproterozoic Era H Calymmian Period a water – d e Ectasian Period a ←Earliest water Stenian Period -4500 — n ←Earth (−4540) (million years ago) Clickable Neoproterozoic Era ( Tonian Period Cryogenian Period Ediacaran Period Phanerozoic Eon Paleozoic Era Cambrian Period Ordovician Period Silurian Period Devonian Period Carboniferous Period Permian Period Mesozoic Era Triassic Period Jurassic Period Cretaceous Period Cenozoic Era Paleogene Period Neogene Period Quaternary Period Etymology of period names References See also External links Dating of the Geologic record The Geologic record is the strata (layers) of rock in the planet's crust and the science of geology is much concerned with the age and origin of all rocks to determine the history and formation of Earth and to understand the forces that have acted upon it. -

Understanding the Causes of Bush Encroachment in Africa: the Key to Effective Management of Savanna Grasslands
Tropical Grasslands – Forrajes Tropicales (2013) Volume 1, 215−219 Understanding the causes of bush encroachment in Africa: The key to effective management of savanna grasslands OLAOTSWE E. KGOSIKOMA1 AND KABO MOGOTSI2 1Department of Agricultural Research, Ministry of Agriculture, Gaborone, Botswana. www.moa.gov.bw 2Department of Agricultural Research, Ministry of Agriculture, Francistown, Botswana. www.moa.gov.bw Keywords: Rangeland degradation, fire, indigenous ecological knowledge, livestock grazing, rainfall variability. Abstract The increase in biomass and abundance of woody plant species, often thorny or unpalatable, coupled with the suppres- sion of herbaceous plant cover, is a widely recognized form of rangeland degradation. Bush encroachment therefore has the potential to compromise rural livelihoods in Africa, as many depend on the natural resource base. The causes of bush encroachment are not without debate, but fire, herbivory, nutrient availability and rainfall patterns have been shown to be the key determinants of savanna vegetation structure and composition. In this paper, these determinants are discussed, with particular reference to arid and semi-arid environments of Africa. To improve our current under- standing of causes of bush encroachment, an integrated approach, involving ecological and indigenous knowledge systems, is proposed. Only through our knowledge of causes of bush encroachment, both direct and indirect, can better livelihood adjustments be made, or control measures and restoration of savanna ecosystem functioning be realized. Resumen Una forma ampliamente reconocida de degradación de pasturas es el incremento de la abundancia de especies de plan- tas leñosas, a menudo espinosas y no palatables, y de su biomasa, conjuntamente con la pérdida de plantas herbáceas. En África, la invasión por arbustos puede comprometer el sistema de vida rural ya que muchas personas dependen de los recursos naturales básicos. -

University of California Santa Cruz Responding to An
UNIVERSITY OF CALIFORNIA SANTA CRUZ RESPONDING TO AN EMERGENT PLANT PEST-PATHOGEN COMPLEX ACROSS SOCIAL-ECOLOGICAL SCALES A dissertation submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in ENVIRONMENTAL STUDIES with an emphasis in ECOLOGY AND EVOLUTIONARY BIOLOGY by Shannon Colleen Lynch December 2020 The Dissertation of Shannon Colleen Lynch is approved: Professor Gregory S. Gilbert, chair Professor Stacy M. Philpott Professor Andrew Szasz Professor Ingrid M. Parker Quentin Williams Acting Vice Provost and Dean of Graduate Studies Copyright © by Shannon Colleen Lynch 2020 TABLE OF CONTENTS List of Tables iv List of Figures vii Abstract x Dedication xiii Acknowledgements xiv Chapter 1 – Introduction 1 References 10 Chapter 2 – Host Evolutionary Relationships Explain 12 Tree Mortality Caused by a Generalist Pest– Pathogen Complex References 38 Chapter 3 – Microbiome Variation Across a 66 Phylogeographic Range of Tree Hosts Affected by an Emergent Pest–Pathogen Complex References 110 Chapter 4 – On Collaborative Governance: Building Consensus on 180 Priorities to Manage Invasive Species Through Collective Action References 243 iii LIST OF TABLES Chapter 2 Table I Insect vectors and corresponding fungal pathogens causing 47 Fusarium dieback on tree hosts in California, Israel, and South Africa. Table II Phylogenetic signal for each host type measured by D statistic. 48 Table SI Native range and infested distribution of tree and shrub FD- 49 ISHB host species. Chapter 3 Table I Study site attributes. 124 Table II Mean and median richness of microbiota in wood samples 128 collected from FD-ISHB host trees. Table III Fungal endophyte-Fusarium in vitro interaction outcomes. -

Noctuoidea: Erebidae: Others
Staude et al. / Metamorphosis 27: S165–S188 S165 ____________________________________________________________________________________________________________________________ Noctuoidea: Erebidae: Others Reference/ Lepidoptera Host plant Locality rearing no. Taxon Subfamily Family Taxon Family M1148 Anoba angulilinea Anobinae Erebidae Dalbergia Fabaceae Tshukudu Game melanoxylon Reserve, Hoedspruit M998 Anoba atripuncta Anobinae Erebidae Ormocarpum Fabaceae Tshukudu Game trichocarpum Reserve, Hoedspruit Gv71 Baniana arvorum Anobinae Erebidae Elephantorrhiza Fabaceae Steenkoppies, farm, elephantina Magaliesburg 14HSS52 Baniana arvorum Anobinae Erebidae Elephantorrhiza Fabaceae Steenkoppies, farm, elephantina Magaliesburg 13HSS84 Plecoptera arctinotata Anobinae Erebidae Senegalia caffra Fabaceae Steenkoppies, farm, Magaliesburg M1020a Plecoptera flaviceps Anobinae Erebidae Dalbergia Fabaceae Casketts, farm, melanoxylon Hoedspruit M317 Bareia incidens Calpinae Erebidae Ficus lutea Moraceae Casketts, farm, (unplaced as to Hoedspruit tribe) 14HSS87 Egnasia vicaria Calpinae Erebidae Afrocanthium Rubiaceae Dlinsa Forest, (unplaced as to mundianum Eshowe tribe) 12HSS163 Exophyla multistriata Calpinae Erebidae Celtis africana Cannabaceae Golden Valley, (unplaced as to Magaliesburg tribe) M416 Exophyla multistriata Calpinae Erebidae Trema orientalis Cannabaceae Sekororo, Tzaneen (unplaced as to (Fed on Celtis tribe) africana) M743 Lacera alope Calpinae Erebidae Pterolobium Fabaceae Moholoholo Rehab (unplaced as to stellatum Centre, Hoedspruit tribe) -

Meet the Gilded Lady 2 Mummies Now Open
Member Magazine Spring 2017 Vol. 42 No. 2 Mummies meet the gilded lady 2 mummies now open Seeing Inside Today, computerized inside of mummies, revealing CT scans of the Gilded Lady tomography (CT) scanning details about the person’s reveal that she was probably offers researchers glimpses age, appearance, and health. in her forties. They also suggest of mummified individuals “Scans like these are noninvasive, that she may have suffered like never before. By combining they’re repeatable, and they from tuberculosis, a common thousands of cross-sectioned can be done without damaging disease at the time. x-ray images, CT scans let the history that we’re trying researchers examine the to understand,” Thomas says. Mummy #30007, known as the Gilded Lady, is one of the most beautifully preserved mummies from The Field Museum’s collection, and one of 19 now on view in the special exhibition Mummies. For decades, keeping mummies like this one well preserved also meant severely limiting the ability of researchers to study them. The result is that little was known about the Gilded Lady beyond what could be gleaned from the mummy’s exterior, with its intricate linen bindings, gilded headdress, and painted facial features. Exterior details do offer some clues. The mummy dates from 30 BC–AD 395, a period when Egypt was a province of the Roman Empire. While the practice of mummification endured in Egypt, it was being transformed by Roman influences. Before the Roman era, for example, mummies had been placed in wooden coffins, while the Gilded Lady is preserved in only linen wrappings and cartonnage, a papier mâché-like material. -

Oligocene and Early Miocene Mammal Biostratigraphy of the Valley of Lakes in Mongolia
Palaeobio Palaeoenv (2017) 97:219–231 DOI 10.1007/s12549-016-0264-x ORIGINAL PAPER Oligocene and early Miocene mammal biostratigraphy of the Valley of Lakes in Mongolia Mathias Harzhauser1 & Gudrun Daxner-Höck1 & Margarita A. Erbajeva2 & Paloma López-Guerrero1,3 & Olivier Maridet4,5 & Adriana Oliver 1,6 & Werner E. Piller7 & Ursula B. Göhlich1 & Reinhard Ziegler8 Received: 13 July 2016 /Revised: 28 October 2016 /Accepted: 10 November 2016 /Published online: 15 December 2016 # The Author(s) 2017. This article is published with open access at Springerlink.com Abstract The Taatsiin Gol Basin in Mongolia is a key area for data. Therefore, we test and evaluate the informal biozonation understanding the evolution and dispersal of Central Asian scheme that has been traditionally used for biostratigraphic mammal faunas during the Oligocene and early Miocene. correlations within the basin. Based on the analysis of the huge After two decades of intense fieldwork, the area is extraordi- dataset, a formalised biostratigraphic scheme is proposed. It narily well sampled and taxonomically well studied, yielding a comprises the Cricetops dormitor Taxon Range Zone large dataset of 19,042 specimens from 60 samples. The spec- (Rupelian), subdivided into the Allosminthus khandae Taxon imens represent 176 species-level and 99 genus-level taxa com- Range Subzone and the Huangomys frequens Abundance prising 135 small mammal species and 47 large mammals. A Subzone, the Amphechinus taatsiingolensis Abundance Zone detailed lithostratigraphy and new magnetostratigraphic and (early Chattian), the Amphechinus major Taxon Range Zone radiometric datings provide an excellent frame for these biotic (late Chattian), subdivided into the Yindirtemys deflexus This article is a contribution to the special issue BThe Valley of Lakes in Mongolia, a key area of Cenozoic mammal evolution and stratigraphy^. -

Response of Wildlife to Bush Thinning on the North Central Freehold Farmlands of Namibia T
Forest Ecology and Management 473 (2020) 118330 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Response of wildlife to bush thinning on the north central freehold farmlands of Namibia T Matti T. Nghikembuaa,b,c, Laurie L. Markera,b, Bruce Brewera,b, Lauri Mehtätaloc, Mark Appiahc,d, ⁎ Ari Pappinenc, a Cheetah Conservation Fund, Otjiwarongo, Namibia b CCF Bush PTY Ltd, Otijwarongo, Namibia c University of Eastern Finland, School of Forest Sciences, Joensuu Campus, Yliopistokatu 7, 80101 Joensuu, Finland d Forestry Research Institute of Ghana (CSIR-FORIG), Kumasi, Ghana ARTICLE INFO ABSTRACT Keywords: Agriculture is considered the backbone of the Namibian economy. However, bush encroachment affects ap- Bush encroachment proximately 45 million hectares of Namibian farmland and in the absence of appropriate restoration measures, Biodiversity negatively affects local biodiversity and the national economy. Bush thinning operations on three freehold farms Restoration were assessed to examine the response of local ungulates (small, medium, large) and predators (meso, large). Carrying capacity Camera traps were used to capture wildlife in bush encroached and previously thinned habitats. We hy- Overgrazing pothesized that thinning would increase the activity of small, medium, and large ungulates, meso and large Bush thinning predators, and that the magnitude of the increase in activity at thinned sites would differ among animal types. Our results revealed that the expected animal captures were not equal – small, medium, and large ungulates were common, large predators were least common; thinned areas had more expected animal captures and overall animal-treatment interactions were almost significant (p = 0.051). -

4.0 Ramoelo 20032018.Indd
© University of Hamburg 2018 All rights reserved Klaus Hess Publishers Göttingen & Windhoek www.k-hess-verlag.de ISBN: 978-3-933117-95-3 (Germany), 978-99916-57-43-1 (Namibia) Language editing: Will Simonson (Cambridge), and Proofreading Pal Translation of abstracts to Portuguese: Ana Filipa Guerra Silva Gomes da Piedade Page desing & layout: Marit Arnold, Klaus A. Hess, Ria Henning-Lohmann Cover photographs: front: Thunderstorm approaching a village on the Angolan Central Plateau (Rasmus Revermann) back: Fire in the miombo woodlands, Zambia (David Parduhn) Cover Design: Ria Henning-Lohmann ISSN 1613-9801 Printed in Germany Suggestion for citations: Volume: Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N. (eds.) (2018) Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions. Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Articles (example): Archer, E., Engelbrecht, F., Hänsler, A., Landman, W., Tadross, M. & Helmschrot, J. (2018) Seasonal prediction and regional climate projections for southern Africa. In: Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions (ed. by Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N.), pp. 14–21, Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Corrections brought to our attention will be published at the following location: http://www.biodiversity-plants.de/biodivers_ecol/biodivers_ecol.php Biodiversity & Ecology Journal of the Division Biodiversity, Evolution and Ecology of Plants, Institute for Plant Science and Microbiology, University of Hamburg Volume 6: Climate change and adaptive land management in southern Africa Assessments, changes, challenges, and solutions Edited by Rasmus Revermann1, Kristin M. -

A Review of Potential Methods for Monitoring Rangeland Degradation in Libya Abdulsalam Al-Bukhari1,2, Stephen Hallett1* and Tim Brewer1
Al-bukhari et al. Pastoralism: Research, Policy and Practice (2018) 8:13 Pastoralism: Research, Policy https://doi.org/10.1186/s13570-018-0118-4 and Practice REVIEW Open Access A review of potential methods for monitoring rangeland degradation in Libya Abdulsalam Al-bukhari1,2, Stephen Hallett1* and Tim Brewer1 Abstract Natural and human factors exert a profound impact on the degradation of rangelands, human effects being the most significant factor in increasing the severity of deterioration. This occurs through agricultural expansion at the expense of rangelands, and with the number of domestic and wildlife animals exceeding the natural carrying capacity. This raises concerns about the ongoing sustainability of these land resources, as well as the sustainability of traditional pastoral land practices. Rangelands require effective management, which is dependent upon accurate and timely monitoring data to support the assessment of rangeland deterioration. Natural rangelands provide one of the significant pillars of support for the Libyan national economy. Despite the important role of rangeland in Libya from both economic and environmental perspectives, the vegetation cover of Libyan rangeland has changed adversely qualitatively and quantitatively over the past four decades. Ground-based observation methods are widely used to assess rangeland degradation in Libya. However, multi-temporal observations are often not integrated nor repeatable, making it difficult for rangeland managers to detect degradation consistently. Field study costs are also significantly high in comparison with their accuracy and reliability, both in terms of the time and resources required. Remote-sensing approaches offer the advantage of spanning large geographical areas with multiple spatial, spectral and temporal resolutions. -

Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义1)
第 41 卷 第 3 期 古 脊 椎 动 物 学 报 pp. 220~229 2003 年 7 月 VERTEBRATA PALASIATICA figs. 1~3 Paraceratherium 在新疆准噶尔盆地 北缘的发现及其意义1) 叶 捷1 孟 津2 吴文裕1 (1 中国科学院古脊椎动物与古人类研究所 北京 100044) (2 美国自然历史博物馆 纽约 10024) 关键词 新疆准噶尔盆地 ,晚渐新世 ,副巨犀 中图法分类号 Q915. 877 2000 年 ,笔者在位于新疆准噶尔盆地北缘的福海县哈拉玛盖乡以南的萨尔多依腊地 区测制乌伦古河组地层剖面时 ,在乌伦古河组和索索泉组之间的一套粗碎屑岩层中发现 了一些哺乳动物化石。其中有孟津在 20004 化石点 (46°35. 779′N ,87°43. 818′E) 发现的一 具副巨犀下颌骨。该下颌保存了这类动物的一些重要特征 ,这些特征对于解决长期以来 人们对于巨犀分类的有关争论以及含化石地层的时代提供了重要信息。 新疆萨尔多依腊的巨犀下颌支和牙齿的形态与 Forster2Cooper (1911 :p. 713 ; 1924 : Fig. 7) 描述的 Bugti 的 Paraceratherium bugtiense 标本在以下几个方面很相似 :1) 下颌水平 支底缘在颊齿列部位向下弯凸 ,其最大深度位于 m1、m2 之间 ;2) 联合部在 p2 前下弯 ;3) p2 之前的联合部上表面呈槽形 ,两侧形成锐脊 ;4) 仅有第一对下门齿 (i1) ,第二、三对门 齿已退化消失 ,该齿呈较长的圆锥形 ,伸向下前方 ,左右门齿基部相靠 ,顶端分离 ,其上无 使用磨蚀痕迹 ,齿根很粗壮 ;5) p2 的形态及 p2 没有被磨蚀的迹象。Forster2Cooper 指出 (1924 ,p. 369) ,他建立的 Paraceratherium 属的很特殊的特征是“a pair of downwardly turned tusks”。换句话说 ,是它具有 1) 下弯的下颌联合部和 2) 较长且呈锥形的第一下门齿。新 疆萨尔多依腊的巨犀在这方面无疑与 Paraceratherium 属是一致的。但它较属型种 P. bugtiense 尺寸大、下颌水平支的相对深度大 ,且 p2 之前的联合部更下弯和背面的凹槽更 深。 自 Forster2Cooper (1911) 创建副巨犀属 ( Paraceratherium) 以来 ,该属的含义多次发生变 化。其原因是 ,在 Bugti 地点发现的巨犀类化石的个体大小相差较大。最初 ,Forster2Cooper 将其中一块尺寸较小、保存较好的下颌作为正型标本记述 ,同时将一块残破的下颌联合 部、一些椎体和肢骨暂时归入了该种。但他指出归入该种的残破的下颌联合部、寰椎和肢 骨相对于正型标本尺寸要大得多 ,可能为雄性个体 ,正型标本则为雌性个体。后来 , Forster2Cooper (1923) 又为大尺寸的寰椎和肢骨建立了新属新种 Baluchitherium osborni ,并认 为该种与 Borissiak 所建立的 Indricotherium turgaicum 的肢骨十分相近 (Forster2Cooper , 1923 : p. 35) 。 1) 国家自然科学基金项目(编号 : 40172010 ,49928201) 资助。 收稿日期 :2003 - 02 - 10 3 期 叶 捷等 : Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义 122 Granger 和 Gregory(1936) 在记述内蒙古发现的 Baluchitherium 时认为 -
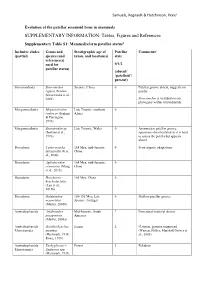
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Timeline of Natural History
Timeline of natural history Main articles: History of the Earth and Geological his- chondrules,[1] are a key signature of a supernova ex- tory of Earth plosion. See also: Geologic time scale and Timeline of evolution- ary history of life • 4,567±3 Ma: Rapid collapse of hydrogen molecular For earlier events, see Timeline of the formation of the cloud, forming a third-generation Population I star, Universe. the Sun, in a region of the Galactic Habitable Zone This timeline of natural history summarizes signifi- (GHZ), about 25,000 light years from the center of the Milky Way Galaxy.[2] • 4,566±2 Ma: A protoplanetary disc (from which Earth eventually forms) emerges around the young Sun, which is in its T Tauri stage. • 4,560–4,550 Ma: Proto-Earth forms at the outer (cooler) edge of the habitable zone of the Solar Sys- tem. At this stage the solar constant of the Sun was only about 73% of its current value, but liquid wa- ter may have existed on the surface of the Proto- Earth, probably due to the greenhouse warming of high levels of methane and carbon dioxide present in the atmosphere. Early bombardment phase begins: because the solar neighbourhood is rife with large planetoids and debris, Earth experiences a number of giant impacts that help to increase its overall size. Visual representation of the history of life on Earth as a spiral 2 Hadean Eon cant geological and biological events from the formation of the Earth to the rise of modern humans. Times are listed in millions of years, or megaanni (Ma).