Rhinoceroses 54 Robin W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Timeline of Natural History
Timeline of natural history This timeline of natural history summarizes significant geological and Life timeline Ice Ages biological events from the formation of the 0 — Primates Quater nary Flowers ←Earliest apes Earth to the arrival of modern humans. P Birds h Mammals – Plants Dinosaurs Times are listed in millions of years, or Karo o a n ← Andean Tetrapoda megaanni (Ma). -50 0 — e Arthropods Molluscs r ←Cambrian explosion o ← Cryoge nian Ediacara biota – z ←Earliest animals o ←Earliest plants i Multicellular -1000 — c Contents life ←Sexual reproduction Dating of the Geologic record – P r The earliest Solar System -1500 — o t Precambrian Supereon – e r Eukaryotes Hadean Eon o -2000 — z o Archean Eon i Huron ian – c Eoarchean Era ←Oxygen crisis Paleoarchean Era -2500 — ←Atmospheric oxygen Mesoarchean Era – Photosynthesis Neoarchean Era Pong ola Proterozoic Eon -3000 — A r Paleoproterozoic Era c – h Siderian Period e a Rhyacian Period -3500 — n ←Earliest oxygen Orosirian Period Single-celled – life Statherian Period -4000 — ←Earliest life Mesoproterozoic Era H Calymmian Period a water – d e Ectasian Period a ←Earliest water Stenian Period -4500 — n ←Earth (−4540) (million years ago) Clickable Neoproterozoic Era ( Tonian Period Cryogenian Period Ediacaran Period Phanerozoic Eon Paleozoic Era Cambrian Period Ordovician Period Silurian Period Devonian Period Carboniferous Period Permian Period Mesozoic Era Triassic Period Jurassic Period Cretaceous Period Cenozoic Era Paleogene Period Neogene Period Quaternary Period Etymology of period names References See also External links Dating of the Geologic record The Geologic record is the strata (layers) of rock in the planet's crust and the science of geology is much concerned with the age and origin of all rocks to determine the history and formation of Earth and to understand the forces that have acted upon it. -

Meet the Gilded Lady 2 Mummies Now Open
Member Magazine Spring 2017 Vol. 42 No. 2 Mummies meet the gilded lady 2 mummies now open Seeing Inside Today, computerized inside of mummies, revealing CT scans of the Gilded Lady tomography (CT) scanning details about the person’s reveal that she was probably offers researchers glimpses age, appearance, and health. in her forties. They also suggest of mummified individuals “Scans like these are noninvasive, that she may have suffered like never before. By combining they’re repeatable, and they from tuberculosis, a common thousands of cross-sectioned can be done without damaging disease at the time. x-ray images, CT scans let the history that we’re trying researchers examine the to understand,” Thomas says. Mummy #30007, known as the Gilded Lady, is one of the most beautifully preserved mummies from The Field Museum’s collection, and one of 19 now on view in the special exhibition Mummies. For decades, keeping mummies like this one well preserved also meant severely limiting the ability of researchers to study them. The result is that little was known about the Gilded Lady beyond what could be gleaned from the mummy’s exterior, with its intricate linen bindings, gilded headdress, and painted facial features. Exterior details do offer some clues. The mummy dates from 30 BC–AD 395, a period when Egypt was a province of the Roman Empire. While the practice of mummification endured in Egypt, it was being transformed by Roman influences. Before the Roman era, for example, mummies had been placed in wooden coffins, while the Gilded Lady is preserved in only linen wrappings and cartonnage, a papier mâché-like material. -

Oligocene and Early Miocene Mammal Biostratigraphy of the Valley of Lakes in Mongolia
Palaeobio Palaeoenv (2017) 97:219–231 DOI 10.1007/s12549-016-0264-x ORIGINAL PAPER Oligocene and early Miocene mammal biostratigraphy of the Valley of Lakes in Mongolia Mathias Harzhauser1 & Gudrun Daxner-Höck1 & Margarita A. Erbajeva2 & Paloma López-Guerrero1,3 & Olivier Maridet4,5 & Adriana Oliver 1,6 & Werner E. Piller7 & Ursula B. Göhlich1 & Reinhard Ziegler8 Received: 13 July 2016 /Revised: 28 October 2016 /Accepted: 10 November 2016 /Published online: 15 December 2016 # The Author(s) 2017. This article is published with open access at Springerlink.com Abstract The Taatsiin Gol Basin in Mongolia is a key area for data. Therefore, we test and evaluate the informal biozonation understanding the evolution and dispersal of Central Asian scheme that has been traditionally used for biostratigraphic mammal faunas during the Oligocene and early Miocene. correlations within the basin. Based on the analysis of the huge After two decades of intense fieldwork, the area is extraordi- dataset, a formalised biostratigraphic scheme is proposed. It narily well sampled and taxonomically well studied, yielding a comprises the Cricetops dormitor Taxon Range Zone large dataset of 19,042 specimens from 60 samples. The spec- (Rupelian), subdivided into the Allosminthus khandae Taxon imens represent 176 species-level and 99 genus-level taxa com- Range Subzone and the Huangomys frequens Abundance prising 135 small mammal species and 47 large mammals. A Subzone, the Amphechinus taatsiingolensis Abundance Zone detailed lithostratigraphy and new magnetostratigraphic and (early Chattian), the Amphechinus major Taxon Range Zone radiometric datings provide an excellent frame for these biotic (late Chattian), subdivided into the Yindirtemys deflexus This article is a contribution to the special issue BThe Valley of Lakes in Mongolia, a key area of Cenozoic mammal evolution and stratigraphy^. -

Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义1)
第 41 卷 第 3 期 古 脊 椎 动 物 学 报 pp. 220~229 2003 年 7 月 VERTEBRATA PALASIATICA figs. 1~3 Paraceratherium 在新疆准噶尔盆地 北缘的发现及其意义1) 叶 捷1 孟 津2 吴文裕1 (1 中国科学院古脊椎动物与古人类研究所 北京 100044) (2 美国自然历史博物馆 纽约 10024) 关键词 新疆准噶尔盆地 ,晚渐新世 ,副巨犀 中图法分类号 Q915. 877 2000 年 ,笔者在位于新疆准噶尔盆地北缘的福海县哈拉玛盖乡以南的萨尔多依腊地 区测制乌伦古河组地层剖面时 ,在乌伦古河组和索索泉组之间的一套粗碎屑岩层中发现 了一些哺乳动物化石。其中有孟津在 20004 化石点 (46°35. 779′N ,87°43. 818′E) 发现的一 具副巨犀下颌骨。该下颌保存了这类动物的一些重要特征 ,这些特征对于解决长期以来 人们对于巨犀分类的有关争论以及含化石地层的时代提供了重要信息。 新疆萨尔多依腊的巨犀下颌支和牙齿的形态与 Forster2Cooper (1911 :p. 713 ; 1924 : Fig. 7) 描述的 Bugti 的 Paraceratherium bugtiense 标本在以下几个方面很相似 :1) 下颌水平 支底缘在颊齿列部位向下弯凸 ,其最大深度位于 m1、m2 之间 ;2) 联合部在 p2 前下弯 ;3) p2 之前的联合部上表面呈槽形 ,两侧形成锐脊 ;4) 仅有第一对下门齿 (i1) ,第二、三对门 齿已退化消失 ,该齿呈较长的圆锥形 ,伸向下前方 ,左右门齿基部相靠 ,顶端分离 ,其上无 使用磨蚀痕迹 ,齿根很粗壮 ;5) p2 的形态及 p2 没有被磨蚀的迹象。Forster2Cooper 指出 (1924 ,p. 369) ,他建立的 Paraceratherium 属的很特殊的特征是“a pair of downwardly turned tusks”。换句话说 ,是它具有 1) 下弯的下颌联合部和 2) 较长且呈锥形的第一下门齿。新 疆萨尔多依腊的巨犀在这方面无疑与 Paraceratherium 属是一致的。但它较属型种 P. bugtiense 尺寸大、下颌水平支的相对深度大 ,且 p2 之前的联合部更下弯和背面的凹槽更 深。 自 Forster2Cooper (1911) 创建副巨犀属 ( Paraceratherium) 以来 ,该属的含义多次发生变 化。其原因是 ,在 Bugti 地点发现的巨犀类化石的个体大小相差较大。最初 ,Forster2Cooper 将其中一块尺寸较小、保存较好的下颌作为正型标本记述 ,同时将一块残破的下颌联合 部、一些椎体和肢骨暂时归入了该种。但他指出归入该种的残破的下颌联合部、寰椎和肢 骨相对于正型标本尺寸要大得多 ,可能为雄性个体 ,正型标本则为雌性个体。后来 , Forster2Cooper (1923) 又为大尺寸的寰椎和肢骨建立了新属新种 Baluchitherium osborni ,并认 为该种与 Borissiak 所建立的 Indricotherium turgaicum 的肢骨十分相近 (Forster2Cooper , 1923 : p. 35) 。 1) 国家自然科学基金项目(编号 : 40172010 ,49928201) 资助。 收稿日期 :2003 - 02 - 10 3 期 叶 捷等 : Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义 122 Granger 和 Gregory(1936) 在记述内蒙古发现的 Baluchitherium 时认为 -
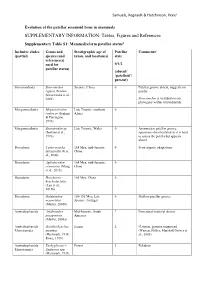
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Timeline of Natural History
Timeline of natural history Main articles: History of the Earth and Geological his- chondrules,[1] are a key signature of a supernova ex- tory of Earth plosion. See also: Geologic time scale and Timeline of evolution- ary history of life • 4,567±3 Ma: Rapid collapse of hydrogen molecular For earlier events, see Timeline of the formation of the cloud, forming a third-generation Population I star, Universe. the Sun, in a region of the Galactic Habitable Zone This timeline of natural history summarizes signifi- (GHZ), about 25,000 light years from the center of the Milky Way Galaxy.[2] • 4,566±2 Ma: A protoplanetary disc (from which Earth eventually forms) emerges around the young Sun, which is in its T Tauri stage. • 4,560–4,550 Ma: Proto-Earth forms at the outer (cooler) edge of the habitable zone of the Solar Sys- tem. At this stage the solar constant of the Sun was only about 73% of its current value, but liquid wa- ter may have existed on the surface of the Proto- Earth, probably due to the greenhouse warming of high levels of methane and carbon dioxide present in the atmosphere. Early bombardment phase begins: because the solar neighbourhood is rife with large planetoids and debris, Earth experiences a number of giant impacts that help to increase its overall size. Visual representation of the history of life on Earth as a spiral 2 Hadean Eon cant geological and biological events from the formation of the Earth to the rise of modern humans. Times are listed in millions of years, or megaanni (Ma). -

Linxia Basin: an Ancient Paradise for Late Cenozoic Rhinoceroses in North China
Vol.24 No.2 2010 Paleomammalogy Linxia Basin: An Ancient Paradise for Late Cenozoic Rhinoceroses in North China DENG Tao * Institute of Vertebrate Paleontology and Paleoanthropology, CAS, Beijing 100044 he Linxia Basin is located and sometimes partially articulated several hundred skulls of the late on the triple-junction of the bones of large mammals, which often Cenozoic rhinoceroses are known from Tnortheastern Tibetan Plateau, occur in dense concentrations. Many the Linxia Basin. In addition, more western Qinling Mountains and the new species of the Late Oligocene abundant limb bones and isolated teeth Loess Plateau. The basin is filled with Dzungariotherium fauna, the Middle of rhinoceroses are found in this basin, 700−2000 m of late Cenozoic deposits, Miocene Platybelodon fauna, the Late especially from the Late Miocene mainly red in color and dominated by Miocene Hipparion fauna, and the Early red clay deposits. Rhinoceroses were lacustrine siltstones and mudstones, and Pleistocene Equus fauna have been over 70% in diversity during the Late the Linxia sequence represents the most described from the Linxia Basin since Oligocene, and they were dominant in complete and successive late Cenozoic 2000, including rodents, lagomorphs, population during the Late Miocene. section in China. The localities in the primates, carnivores, proboscideans, In the Middle Miocene and Early Linxia Basin are notable for abundant, perissodactyls and artiodactyls. Pleistocene faunas, rhinoceroses were relatively complete, well-preserved, Among these mammalian fossils, important members. Late Oligocene The Late Oligocene fauna of findings in Europe (Qiu and Wang, is estimated at 24 tons, and another the Linxia Basin comes from the 2007). -

Managing Woody Bush Encroachment Impacting
MANAGING WOODY BUSH ENCROACHMENT IMPACTING SOUTHERN WHITE RHINO (CERATOTHERIUM SIMUM SIMUM) HABITAT ON A PRIVATE RESERVE IN SOUTH AFRICA by Kelli Ann Stephens A Thesis Submitted in partial fulfillment of the requirements for the degree Master of Environmental Studies The Evergreen State College June 2019 ©2019 by Kelli Ann Stephens. All rights reserved. This Thesis for the Master of Environmental Studies Degree by Kelli Ann Stephens has been approved for The Evergreen State College by ________________________ Tyrus Smith, PhD Member of the Faculty ________________________ Date ABSTRACT Managing woody bush encroachment impacting southern white rhino (Ceratotherium simum simum) habitat on a private reserve in South Africa Kelli Ann Stephens Private nature reserves in South Africa have been a key contributor to the stabilization of southern white rhino (Ceratotherium simum simum) populations. However, woody bush encroachment is a widespread problem threatening the vital grasslands these rhinos rely on. This research aims to help UmPhafa Private Nature Reserve establish a bush encroachment management plan by identifying land cover changes over the past 18 years and by establishing key areas to restore to grasslands. UmPhafa’s white rhino population was observed for six weeks during the summer months (Jan.-Feb, 2019) where limited- species quadrat surveys acquired the data on lemon bush (Lippia javanica), scented-pod acacia (Vachellia nilotica), sweet-thorn acacia (Vachellia karroo), paperbark acacia (Vachellia sieberiana var. woodii), and hook-thorn acacia (Senegalia caffra) concentrations near the rhino’s summer habitat. These maps were combined with hotspot encroachment locations, ranger encroachment maps, and past seasonal rhino movements by to establish ten zones that encompassed 144.5 hectares for restoration. -

MERICAN MUSEUM NOVITATES Published by Number 78 the AMERICAN MUSEUM of NATURAL HISTORY May 25, 1923 New York City
-MERICAN MUSEUM NOVITATES Published by Number 78 THE AMERICAN MUSEUM OF NATURAL HISTORY May 25, 1923 New York City 56.9,72B(51.7) BALUCHITHERIUM GRANGERI, A GIANT HORNLESS RHINOCEROS FROM MONGOLIA BY HENRY FAIRFIELD OSBORN' In previous communications on the rhinoceroses (Rhinoceros Con- tributions 1 to 11), Osborn separated six distinct phyla or subfamilies. The remarkable discoveries by Clive Forster Cooper in Baluchistan, by A. Borissiak in north Turkestan, and by Walter Granger of the Third Asiatic Expedition in southeastern and central Mongolia, indicate the existence of a seventh subfamily which we may term Baluchitheriinw, if the generic name proves valid. At present our knowledge rests on the following materials: BUGTI HILLS, Chur-lando, Baluchistan. Cooper Collection, British Museum. Paraceratherium bugtiense Cooper, December 1911. Fairly complete skulls and lower jaws of about the size of a large rhinoceros, simple aceratherine molars, abnormal lower incisors. Y'haumastotherium osborni Cooper, October 1913, changed to Baluchitherium osborni Cooper, November 1913. Fragmentary skeletal remains found in close proximity to Paraceratherium, including neck vertebrae, foot and limb bones of elephantine size. TURGAI, a province of north Turkestan. Discoveries by A. Borissiak, published 1915-1918. Indricotherium asiaticum Borissiak, 1916. Teeth, skull, and skeletal remains, occurring in situ and resembling both Paraceratherium and Baluchitherium. Epiaceratherium turgaicum Borissiak, 1918.2 LOH, central Mongolia, Third Asiatic Expedition Collection, 1922. Associated skull and skeletal remains similar in size to the type of Baluchitherium osborni. Baluchitherium grangeri, new species. Type, nearly complete skull and jaws (Amer. Mus. 18650) associated with parts of vertebrae and of limb bones, as described in the present bulletin. -

Oligocene Ruminants from the Kızılırmak Formation, Çankırı-Çorum Basin, Central Anatolia, Turkey
Palaeontologia Electronica palaeo-electronica.org Oligocene ruminants from the Kızılırmak Formation, Çankırı-Çorum Basin, Central Anatolia, Turkey Grégoire Métais, Ebru Albayrak, Pierre-Olivier Antoine, Ozan Erdal, Levent Karadenizli, Neşe Oyal, Gerçek Saraç, Yeşim Büyükmeriç, and Sevket Sen ABSTRACT A new assemblage of ruminants from five distinct Oligocene localities of the Kızılırmak Formation, Central Anatolia, Turkey is described. The tragulids Iberomeryx parvus, and Iberomeryx sp. (large), as well as a probable large lophiomerycid have been recognized. The stem pecoran Dremotherium guthi, cf. Palaeohypsodontus and a large indeterminate Pecora have been identified as well. In the five localities, the majority of the ruminant material is referred to Iberomeryx parvus, but the sample from the locality Tepe 641 (upper member of the Kızılırmak Formation) shows some distinc- tive characters suggesting a more derived species/forms that probably lived in more open environments. The ruminant taxa recorded in the Kızılırmak Formation are con- gruent with a late Oligocene age, probably close in age to the Benara fauna of south- ern Georgia. The possible occurrence of Palaeohypsodontus in Central Anatolia would significantly expand its geographical range and suggest biogeographical affinities with Central Asia. The ruminant fauna from the Kızılırmak Formation suggests the exis- tence of lowland forests with more open landscapes in central Anatolia during the late Oligocene. Grégoire Métais. Sorbonne Universités, CR2P, MNHN, CNRS, UPMC, Muséum National d’Histoire Naturelle, 57 rue Cuvier, CP 38, 75005 Paris, France. [email protected]. Ebru Albayrak. Natural History Museum, Maden ve Tektik Arama Genel Müdürlügü, 06520 Ankara, Turkey, [email protected] Pierre-Olivier Antoine. ISEM, Université de Montpellier, CNRS, IRD, EPHE, CC64, Place Eugène Bataillon, F-34095 Montpellier, France, [email protected] Ozan Erdal. -

Paleontology 9: Cenozoic
PALEONTOLOGY 9: CENOZOIC VOLUME 9, ISSUE 9, SEPTEMBER 2020 AGE OF MAMMALS THIS MONTH This issue on the Cenozoic Era • Cenozoic Dioramas page 2 is the last in the Paleontology series. The Cenozoic Era • Complete Your Timeline! page 9 covers the time from the end of dinosaurs to present day. • DNA Mutations • Build Models page 19 Over the course of this series, • Bit of Wobble page 26 we have included information • Meiosis page 33 and activities on DNA. DNA, the • Lethal Gene page 35 blueprint for all life, is the • Mutations page 43 foundation of biology. A fundamental understanding of Megatherium americanum is the POWER WORDS DNA helps us to understand largest ground sloth. It was 30 feet, • Cenozoic Era: current how species originate, have a and lived from 400,000 to 8,000 years and most recent of the 3 range of existence, and go ago in Argentina, Bolivia, and Uruguay. geological eras of the extinct. This issue will complete Phanerozoic Eon; it the DNA activities with are still here and doing well in follows the Mesozoic explorations of how DNA the tropics of South and Central Era 66 MYA to present mutates, and the consequences America! • Eocene: an Epoch in of mutations. the Cenozoic Era from The Cenozoic Era is the Age of 56 to 34 MYA My scientific research is on the Mammals. North America was • Epoch: geologic time mammal group xenarthrans home to many species of saber- within a Period (ground and tree sloths, toothed cats, dire wolves, short- anteaters, armadillos, faced bears, terror birds, CAREER CONNECTION glyptodonts, and pampatheres). -

Why Sauropods Had Long Necks; and Why Giraffes Have Short Necks
TAYLOR AND WEDEL – LONG NECKS OF SAUROPOD DINOSAURS 1 of 39 Why sauropods had long necks; and why giraffes have short necks Michael P. Taylor, Department of Earth Sciences, University of Bristol, Bristol BS8 1RJ, England. [email protected] Mathew J. Wedel, College of Osteopathic Medicine of the Pacific and College of Podiatric Medicine, Western University of Health Sciences, 309 E. Second Street, Pomona, California 91766-1854, USA. [email protected] Table of Contents Abstract............................................................................................................................................2 Introduction......................................................................................................................................3 Museum Abbreviations...............................................................................................................3 Long Necks in Different Taxa..........................................................................................................3 Extant Animals............................................................................................................................4 Extinct Mammals........................................................................................................................4 Theropods....................................................................................................................................5 Pterosaurs....................................................................................................................................6