Oligocene and Early Miocene Mammal Biostratigraphy of the Valley of Lakes in Mongolia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Timeline of Natural History
Timeline of natural history This timeline of natural history summarizes significant geological and Life timeline Ice Ages biological events from the formation of the 0 — Primates Quater nary Flowers ←Earliest apes Earth to the arrival of modern humans. P Birds h Mammals – Plants Dinosaurs Times are listed in millions of years, or Karo o a n ← Andean Tetrapoda megaanni (Ma). -50 0 — e Arthropods Molluscs r ←Cambrian explosion o ← Cryoge nian Ediacara biota – z ←Earliest animals o ←Earliest plants i Multicellular -1000 — c Contents life ←Sexual reproduction Dating of the Geologic record – P r The earliest Solar System -1500 — o t Precambrian Supereon – e r Eukaryotes Hadean Eon o -2000 — z o Archean Eon i Huron ian – c Eoarchean Era ←Oxygen crisis Paleoarchean Era -2500 — ←Atmospheric oxygen Mesoarchean Era – Photosynthesis Neoarchean Era Pong ola Proterozoic Eon -3000 — A r Paleoproterozoic Era c – h Siderian Period e a Rhyacian Period -3500 — n ←Earliest oxygen Orosirian Period Single-celled – life Statherian Period -4000 — ←Earliest life Mesoproterozoic Era H Calymmian Period a water – d e Ectasian Period a ←Earliest water Stenian Period -4500 — n ←Earth (−4540) (million years ago) Clickable Neoproterozoic Era ( Tonian Period Cryogenian Period Ediacaran Period Phanerozoic Eon Paleozoic Era Cambrian Period Ordovician Period Silurian Period Devonian Period Carboniferous Period Permian Period Mesozoic Era Triassic Period Jurassic Period Cretaceous Period Cenozoic Era Paleogene Period Neogene Period Quaternary Period Etymology of period names References See also External links Dating of the Geologic record The Geologic record is the strata (layers) of rock in the planet's crust and the science of geology is much concerned with the age and origin of all rocks to determine the history and formation of Earth and to understand the forces that have acted upon it. -

LETTER Doi:10.1038/Nature14423
LETTER doi:10.1038/nature14423 A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings Xing Xu1,2*, Xiaoting Zheng1,3*, Corwin Sullivan2, Xiaoli Wang1, Lida Xing4, Yan Wang1, Xiaomei Zhang3, Jingmai K. O’Connor2, Fucheng Zhang2 & Yanhong Pan5 The wings of birds and their closest theropod relatives share a ratios are 1.16 and 1.08, respectively, compared to 0.96 and 0.78 in uniform fundamental architecture, with pinnate flight feathers Epidendrosaurus and 0.79 and 0.66 in Epidexipteryx), an extremely as the key component1–3. Here we report a new scansoriopterygid short humeral deltopectoral crest, and a long rod-like bone articu- theropod, Yi qi gen. et sp. nov., based on a new specimen from the lating with the wrist. Middle–Upper Jurassic period Tiaojishan Formation of Hebei Key osteological features are as follows. STM 31-2 (Fig. 1) is inferred Province, China4. Yi is nested phylogenetically among winged ther- to be an adult on the basis of the closed neurocentral sutures of the opods but has large stiff filamentous feathers of an unusual type on visible vertebrae, although this is not a universal criterion for maturity both the forelimb and hindlimb. However, the filamentous feath- across archosaurian taxa12. Its body mass is estimated to be approxi- ers of Yi resemble pinnate feathers in bearing morphologically mately 380 g, using an empirical equation13. diverse melanosomes5. Most surprisingly, Yi has a long rod-like The skull and mandible are similar to those of other scansoriopter- bone extending from each wrist, and patches of membranous tissue ygids, and to a lesser degree to those of oviraptorosaurs and some basal preserved between the rod-like bones and the manual digits. -

Erinaceidae, Insectivora, Mammalia) from the Oligocene of Mongolia A
Paleontological Journal, Vol. 36, No. 3, 2002, pp. 302–306. Translated from Paleontologicheskii Zhurnal, No. 3, 2002, pp. 75–80. Original Russian Text Copyright © 2002 by Lopatin. English Translation Copyright © 2002 by åÄIä “Nauka /Interperiodica” (Russia). The Largest Asiatic Amphechinus (Erinaceidae, Insectivora, Mammalia) from the Oligocene of Mongolia A. V. Lopatin Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya ul. 123, Moscow, 117997 Russia e-mail: [email protected] Received October 26, 2000 Abstract—A lower jaw fragment of a new hedgehog species, Amphechinus gigas sp. nov., from the Oligocene Shand-Gol Formation of Mongolia is described. This species is substantially larger than A. rectus, A. akespensis, and other known Amphechinus species from Asia and comparable in size to the European species A. robustus, A. ginsburgi, and A. intermedius. Regarding the length of the lower cheek tooth row, A. gigas is comparable to Recent Erinaceus europaeus; however, the much deeper and more massive horizontal ramus of the dentary shows that A. gigas is larger than the latter. INTRODUCTION europaeus; at the same time, its lower jaw is substan- tially larger and more massive than those of the listed Amphechinus belongs to the earliest genera of the species. The fragmentary lower jaw of the new species subfamily Erinaceinae. This genus was widespread in was found together with A. rectus and A. cf. kansuensis. the Oligocene and Miocene of Eurasia and occurred in the Miocene of North America and Africa (Gureev, 1979; Gould, 1995). The following six species were described from Asia: Oligocene A. rectus (Matthew et Granger, 1924), A. kansuensis (Bohlin, 1942), and A. -

Catalogue Palaeontology Vertebrates (Updated July 2020)
Hermann L. Strack Livres Anciens - Antiquarian Bookdealer - Antiquariaat Histoire Naturelle - Sciences - Médecine - Voyages Sciences - Natural History - Medicine - Travel Wetenschappen - Natuurlijke Historie - Medisch - Reizen Porzh Hervé - 22780 Loguivy Plougras - Bretagne - France Tel.: +33-(0)679439230 - email: [email protected] site: www.strackbooks.nl Dear friends and customers, I am pleased to present my new catalogue. Most of my book stock contains many rare and seldom offered items. I hope you will find something of interest in this catalogue, otherwise I am in the position to search any book you find difficult to obtain. Please send me your want list. I am always interested in buying books, journals or even whole libraries on all fields of science (zoology, botany, geology, medicine, archaeology, physics etc.). Please offer me your duplicates. Terms of sale and delivery: We accept orders by mail, telephone or e-mail. All items are offered subject to prior sale. Please do not forget to mention the unique item number when ordering books. Prices are in Euro. Postage, handling and bank costs are charged extra. Books are sent by surface mail (unless we are instructed otherwise) upon receipt of payment. Confirmed orders are reserved for 30 days. If payment is not received within that period, we are in liberty to sell those items to other customers. Return policy: Books may be returned within 14 days, provided we are notified in advance and that the books are well packed and still in good condition. Catalogue Palaeontology Vertebrates (Updated July 2020) Archaeology AE11189 ROSSI, M.S. DE, 1867. € 80,00 Rapporto sugli studi e sulle scoperte paleoetnologiche nel bacino della campagna romana del Cav. -

Chapter 1 - Introduction
EURASIAN MIDDLE AND LATE MIOCENE HOMINOID PALEOBIOGEOGRAPHY AND THE GEOGRAPHIC ORIGINS OF THE HOMININAE by Mariam C. Nargolwalla A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Anthropology University of Toronto © Copyright by M. Nargolwalla (2009) Eurasian Middle and Late Miocene Hominoid Paleobiogeography and the Geographic Origins of the Homininae Mariam C. Nargolwalla Doctor of Philosophy Department of Anthropology University of Toronto 2009 Abstract The origin and diversification of great apes and humans is among the most researched and debated series of events in the evolutionary history of the Primates. A fundamental part of understanding these events involves reconstructing paleoenvironmental and paleogeographic patterns in the Eurasian Miocene; a time period and geographic expanse rich in evidence of lineage origins and dispersals of numerous mammalian lineages, including apes. Traditionally, the geographic origin of the African ape and human lineage is considered to have occurred in Africa, however, an alternative hypothesis favouring a Eurasian origin has been proposed. This hypothesis suggests that that after an initial dispersal from Africa to Eurasia at ~17Ma and subsequent radiation from Spain to China, fossil apes disperse back to Africa at least once and found the African ape and human lineage in the late Miocene. The purpose of this study is to test the Eurasian origin hypothesis through the analysis of spatial and temporal patterns of distribution, in situ evolution, interprovincial and intercontinental dispersals of Eurasian terrestrial mammals in response to environmental factors. Using the NOW and Paleobiology databases, together with data collected through survey and excavation of middle and late Miocene vertebrate localities in Hungary and Romania, taphonomic bias and sampling completeness of Eurasian faunas are assessed. -

Meet the Gilded Lady 2 Mummies Now Open
Member Magazine Spring 2017 Vol. 42 No. 2 Mummies meet the gilded lady 2 mummies now open Seeing Inside Today, computerized inside of mummies, revealing CT scans of the Gilded Lady tomography (CT) scanning details about the person’s reveal that she was probably offers researchers glimpses age, appearance, and health. in her forties. They also suggest of mummified individuals “Scans like these are noninvasive, that she may have suffered like never before. By combining they’re repeatable, and they from tuberculosis, a common thousands of cross-sectioned can be done without damaging disease at the time. x-ray images, CT scans let the history that we’re trying researchers examine the to understand,” Thomas says. Mummy #30007, known as the Gilded Lady, is one of the most beautifully preserved mummies from The Field Museum’s collection, and one of 19 now on view in the special exhibition Mummies. For decades, keeping mummies like this one well preserved also meant severely limiting the ability of researchers to study them. The result is that little was known about the Gilded Lady beyond what could be gleaned from the mummy’s exterior, with its intricate linen bindings, gilded headdress, and painted facial features. Exterior details do offer some clues. The mummy dates from 30 BC–AD 395, a period when Egypt was a province of the Roman Empire. While the practice of mummification endured in Egypt, it was being transformed by Roman influences. Before the Roman era, for example, mummies had been placed in wooden coffins, while the Gilded Lady is preserved in only linen wrappings and cartonnage, a papier mâché-like material. -

Title a Preliminary Report on Carnivorous Mammals From
A preliminary report on carnivorous mammals from Pondaung Title fauna Author(s) Egi, Naoko; Tsubamoto, Takehisa Citation Asian paleoprimatology (2000), 1: 103-114 Issue Date 2000 URL http://hdl.handle.net/2433/199738 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Asian Paleoprimatology, vol. 1:103-114 (2000) Kyoto University Primate Research Institute A preliminary rer r rt on canlivorous mammals from Pondaung fauna Naoko Egil and Takehisa Tsubamoto2 'Departmentof Geology, NationalScience Museum of Japan, Tokyo 169-0073, Japan 'Departmentof Geologyand Mineralogy , GraduateSchool of Science,Kyoto University, Kyoto606-8502, Japan Abstract Somecarnivore materials have been discovered from the PondaungFormation in cen- tralMyanmar recently. The materials are separableinto at leasttwo genera, both of which are hyaenodontidcreodonts. One of themis a medium-sizedproviverrine. Collected parts in- cludea maxilla,lower molar fragmen'ts, and some postcranial fragments. It showssome distinctivedental characters such as smallprotocone lobe on P4, anterolingually-placed proto- coneand posterolingually-placed metacone relative to paraconeon M' andM2, and relatively large 1\43 with a veryreduced metaconid. The otheris a muchlarger form. A maxillary fragmentwith a M' anda mandibularfraarneliL with P2 to M2have been found for this form. possessessome similarities to Pterodon,but observationson morecomplete specimens all!: comparisonswith other Pterodon-like hyaenodontids from Asia are necessaryto settlea sys- tematicassignment of thisform. The two hyaenodontidsare the onlyknown mammalian predatorsfrom Pondaung fauna (latest Middle Eocene) based on the currentknowledge. Since early in this century, many vertebrate fossils have been collected from the Pondaung Formation (latest Middle Eocene; central Myanmar). Mammals known from the Pondaung fauna belong to a variety of taxa: Artiodactyla, Creodonta, Perissodactyla, Primates, and Rodentia (Takai et al., 1999). -

Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology
Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology by Paul David Polly B.A. (University of Texas at Austin) 1987 A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Paleontology in the GRADUATE DIVISION of the UNIVERSITY of CALIFORNIA at BERKELEY Committee in charge: Professor William A. Clemens, Chair Professor Kevin Padian Professor James L. Patton Professor F. Clark Howell 1993 Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology © 1993 by Paul David Polly To P. Reid Hamilton, in memory. iii TABLE OF CONTENTS Introduction ix Acknowledgments xi Chapter One--Revolution and Evolution in Taxonomy: Mammalian Classification Before and After Darwin 1 Introduction 2 The Beginning of Modern Taxonomy: Linnaeus and his Predecessors 5 Cuvier's Classification 10 Owen's Classification 18 Post-Darwinian Taxonomy: Revolution and Evolution in Classification 24 Kovalevskii's Classification 25 Huxley's Classification 28 Cope's Classification 33 Early 20th Century Taxonomy 42 Simpson and the Evolutionary Synthesis 46 A Box Model of Classification 48 The Content of Simpson's 1945 Classification 50 Conclusion 52 Acknowledgments 56 Bibliography 56 Figures 69 Chapter Two: Hyaenodontidae (Creodonta, Mammalia) from the Early Eocene Four Mile Fauna and Their Biostratigraphic Implications 78 Abstract 79 Introduction 79 Materials and Methods 80 iv Systematic Paleontology 80 The Four Mile Fauna and Wasatchian Biostratigraphic Zonation 84 Conclusion 86 Acknowledgments 86 Bibliography 86 Figures 87 Chapter Three: A New Genus Eurotherium (Creodonta, Mammalia) in Reference to Taxonomic Problems with Some Eocene Hyaenodontids from Eurasia (With B. Lange-Badré) 89 Résumé 90 Abstract 90 Version française abrégéé 90 Introduction 93 Acknowledgments 96 Bibliography 96 Table 3.1: Original and Current Usages of Genera and Species 99 Table 3.2: Species Currently Included in Genera Discussed in Text 101 Chapter Four: The skeleton of Gazinocyon vulpeculus n. -

Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义1)
第 41 卷 第 3 期 古 脊 椎 动 物 学 报 pp. 220~229 2003 年 7 月 VERTEBRATA PALASIATICA figs. 1~3 Paraceratherium 在新疆准噶尔盆地 北缘的发现及其意义1) 叶 捷1 孟 津2 吴文裕1 (1 中国科学院古脊椎动物与古人类研究所 北京 100044) (2 美国自然历史博物馆 纽约 10024) 关键词 新疆准噶尔盆地 ,晚渐新世 ,副巨犀 中图法分类号 Q915. 877 2000 年 ,笔者在位于新疆准噶尔盆地北缘的福海县哈拉玛盖乡以南的萨尔多依腊地 区测制乌伦古河组地层剖面时 ,在乌伦古河组和索索泉组之间的一套粗碎屑岩层中发现 了一些哺乳动物化石。其中有孟津在 20004 化石点 (46°35. 779′N ,87°43. 818′E) 发现的一 具副巨犀下颌骨。该下颌保存了这类动物的一些重要特征 ,这些特征对于解决长期以来 人们对于巨犀分类的有关争论以及含化石地层的时代提供了重要信息。 新疆萨尔多依腊的巨犀下颌支和牙齿的形态与 Forster2Cooper (1911 :p. 713 ; 1924 : Fig. 7) 描述的 Bugti 的 Paraceratherium bugtiense 标本在以下几个方面很相似 :1) 下颌水平 支底缘在颊齿列部位向下弯凸 ,其最大深度位于 m1、m2 之间 ;2) 联合部在 p2 前下弯 ;3) p2 之前的联合部上表面呈槽形 ,两侧形成锐脊 ;4) 仅有第一对下门齿 (i1) ,第二、三对门 齿已退化消失 ,该齿呈较长的圆锥形 ,伸向下前方 ,左右门齿基部相靠 ,顶端分离 ,其上无 使用磨蚀痕迹 ,齿根很粗壮 ;5) p2 的形态及 p2 没有被磨蚀的迹象。Forster2Cooper 指出 (1924 ,p. 369) ,他建立的 Paraceratherium 属的很特殊的特征是“a pair of downwardly turned tusks”。换句话说 ,是它具有 1) 下弯的下颌联合部和 2) 较长且呈锥形的第一下门齿。新 疆萨尔多依腊的巨犀在这方面无疑与 Paraceratherium 属是一致的。但它较属型种 P. bugtiense 尺寸大、下颌水平支的相对深度大 ,且 p2 之前的联合部更下弯和背面的凹槽更 深。 自 Forster2Cooper (1911) 创建副巨犀属 ( Paraceratherium) 以来 ,该属的含义多次发生变 化。其原因是 ,在 Bugti 地点发现的巨犀类化石的个体大小相差较大。最初 ,Forster2Cooper 将其中一块尺寸较小、保存较好的下颌作为正型标本记述 ,同时将一块残破的下颌联合 部、一些椎体和肢骨暂时归入了该种。但他指出归入该种的残破的下颌联合部、寰椎和肢 骨相对于正型标本尺寸要大得多 ,可能为雄性个体 ,正型标本则为雌性个体。后来 , Forster2Cooper (1923) 又为大尺寸的寰椎和肢骨建立了新属新种 Baluchitherium osborni ,并认 为该种与 Borissiak 所建立的 Indricotherium turgaicum 的肢骨十分相近 (Forster2Cooper , 1923 : p. 35) 。 1) 国家自然科学基金项目(编号 : 40172010 ,49928201) 资助。 收稿日期 :2003 - 02 - 10 3 期 叶 捷等 : Paraceratherium 在新疆准噶尔盆地北缘的发现及其意义 122 Granger 和 Gregory(1936) 在记述内蒙古发现的 Baluchitherium 时认为 -
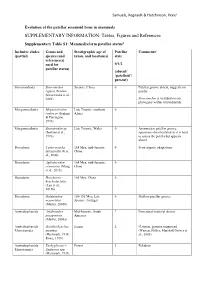
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Zhuding Qiu & Gerhard Storch Contents Introduction Extensive
China Zhuding Qiu & Gerhard Storch Qiu, Z.D. & Storch, G. China. In: Hoek Ostende, L.W. van den, Doukas, C.S. & Reumer, J.W.F. (eds), The Fossil Record of the Eurasian Neogene Insectivores (Erinaceomorpha, Soricomorpha, Mammalia), Part I. Scripta Geologica Special Issue, 5: 37-50, Leiden, November 2005. Z. Qiu, Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Academia Sinica, P.O. Box 643, Beijing 100044, China, ([email protected]); G. Storch, Forschungsinstitut Senckenberg, Senckenberg- Anlage 25, D-60325 Frankfurt am Main, Germany ([email protected]). Contents Introduction .............................................................................................................................................................. 37 Insectivore faunas in the Neogene of China ......................................................................................... 38 References .................................................................................................................................................................. 48 Introduction Extensive excavation activities by the Institute of Vertebrate Paleontology and Paleo- anthropology, Beijing (IVPP) over the last 20 years have increased our knowledge of the Neogene micromammals from China considerably. Yet, it is still rather fragmentary; we deal with a very vast country with a complex geological and faunal history and varied ecological conditions at all times. The fossil sites are not distributed evenly in time and space (Fig. 1), and among