Incresync, INN-Alogliptin+Pioglitazone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
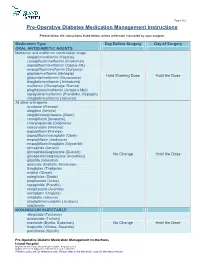
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

NESINA (Alogliptin) Tablets, for Oral Use • Heart Failure: Consider the Risks and Benefits of NESINA Prior to Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use • Acute pancreatitis: There have been postmarketing reports of NESINA safely and effectively. See full prescribing information for acute pancreatitis. If pancreatitis is suspected, promptly NESINA. discontinue NESINA. (5.1) NESINA (alogliptin) tablets, for oral use • Heart failure: Consider the risks and benefits of NESINA prior to Initial U.S. Approval: 2013 initiating treatment in patients at risk for heart failure. If heart failure develops, evaluate and manage according to current ---------------------------RECENT MAJOR CHANGES-------------------------- standards of care and consider discontinuation of NESINA (5.2). Indications and Usage (1.1) 4/2016 • Hypersensitivity: There have been postmarketing reports of Dosage and Administration serious hypersensitivity reactions in patients treated with NESINA Patients with Renal Impairment (2.2) 4/2016 such as anaphylaxis, angioedema and severe cutaneous adverse Warnings and Precautions reactions, including Stevens-Johnson syndrome. In such cases, Pancreatitis (5.1) 4/2016 promptly discontinue NESINA, assess for other potential causes, Heart Failure (5.2) 4/2016 institute appropriate monitoring and treatment and initiate Hepatic Effects (5.4) 4/2016 alternative treatment for diabetes. (5.3) Bullous Pemphigoid (5.7) 12/2016 • Hepatic effects: Postmarketing reports of hepatic failure, ----------------------------INDICATIONS AND USAGE--------------------------- sometimes fatal. Causality cannot be excluded. If liver injury is NESINA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an detected, promptly interrupt NESINA and assess patient for adjunct to diet and exercise to improve glycemic control in adults with probable cause, then treat cause if possible, to resolution or type 2 diabetes mellitus. -

Diabetes Recommendations and Tier Coverage Chart
DIABETES RECOMMENDATIONS AND TIER COVERAGE CHART The American Diabetes Association guidelines for 2020, recommend metformin as the preferred initial treatment for type 2 diabetes (T2DM) along with weight management and physical activity. In patients who have established ASVD or at high risk, CKD, or HF, a SGLT2i or GLP-1 receptor with proven efficacy is recommended independent of A1C. • ASCVD dominates: o GLP-1RA with proven CVD benefit (dulaglutide, liraglutide, injectable semaglutide) OR o SGLT2i with proven CVD benefit (canagliflozin, empagliflozin) if adequate eGFR • HF or CKD dominates: o SGLT2i with evidence of reducing HF and/or CKD progression (empagliflozin, canagliflozin, dapagliflozin) if adequate eGFR OR o If SGLT2i intolerant/contraindicated or eGFR is inadequate, then GLP-1RA with proven CVD benefit In individuals without established cardiovascular disease, pharmacological treatment should be patient-centered taking into account side-effects, cost, impact on weight, risk of hypoglycemia, and other patient preferences. For more detailed information regarding ADA recommendations for pharmacological agents to treat T2DM click here. The following chart is a list of oral and injectable diabetes medications listed by class with their respective A1C reduction and insurance coverage and/or coverage requirements for BCBS, HPHC, Tufts, TMP, and MassHealth. Tufts Medicare Medications BCBSMA HPHC Tufts Preferred MassHealth Biguanides A1C reduction: 1-1.5% metformin Tier 1 Tier 1;2 Tier 1 Tier 1 Covered Glucoghage (metformin) NC NC NC;Tier -

Comparison of Glimepiride, Alogliptin and Alogliptin+Pioglitazone Combination in Poorly Controlled Type 2 Diabetic Patients ( Protocol: Takeda ALO-IIT)
Takeda_ALO-IIT_Ver 3.3 date: 29/Dec/2016 Comparison of glimepiride, alogliptin and alogliptin+pioglitazone combination in poorly controlled type 2 diabetic patients ( Protocol: Takeda_ALO-IIT) Version No: 3.3 date:29/Dec/2016 Principal Investigator’s Affiliation: Seoul National University Bundang Hospital 1 Takeda_ALO-IIT_Ver 3.3 date: 29/Dec/2016 Principal Investigator’s Name: Sung Hee Choi Research Outline Comparison of glimepiride, alogliptin and alogliptin+pioglitazone combination in Title of Research poorly controlled type 2 diabetic patients Principal Investigator Professor Sung Hee Choi Institution Supporting Takeda Pharmaceuticals Korea Co. Ltd. Research Expenses The primary objective is to compare the change in HbA1c in week 24 in 3 treatment groups: the glimepiride monotherapy treatment group; the alogliptin monotherapy treatment group; the alogliptin - pioglitazone combination therapy treatment group. - The secondary objective is to compare the change in HbA1c in week 12 and fasting plasma glucose (FPG) in week 12 and 24 in the following 3 treatment groups over the course of 3 months (at the Baseline, in Week 12): (the glimepiride Research Objective monotherapy treatment group, the alogliptin monotherapy treatment group, and the alogliptin - pioglitazone combination therapy treatment group). - Also, the change in parameters of glycemic variability assessed by CGM will be investigated. - Also, for a 6-month period, the average change in the lipid profile will be compared (Baseline, Week 12, Week 24). · This trial is a three-armed, open label, random assignment trial. · The research subjects are patients who are first starting their treatment or patients who have failed with the metformin treatment and are changing their medication. -

Initial Combination Therapy with Canagliflozin Plus Metformin Versus Each Component As Monotherapy for Drug-Naïve Type 2 Diabe
Diabetes Care Volume 39, March 2016 353 Julio Rosenstock,1 Leonard Chuck,2 Initial Combination Therapy With Manuel Gonzalez-Ortiz,´ 3 Kate Merton,4 CLIN CARE/EDUCATION/NUTRITION/PSYCHOSOCIAL Jagriti Craig,4 George Capuano,4 and Canagliflozin Plus Metformin Rong Qiu4 Versus Each Component as Monotherapy for Drug-Na¨ıve Type 2 Diabetes Diabetes Care 2016;39:353–362 | DOI: 10.2337/dc15-1736 OBJECTIVE This study assessed the efficacy/safety of canagliflozin (CANA), a sodium–glucose cotransporter 2 (SGLT2) inhibitor, plus metformin extended-release (MET) initial therapy in drug-na¨ıve type 2 diabetes. RESEARCH DESIGN AND METHODS This 26-week, double-blind, phase 3 study randomized 1,186 patients to CANA 100 mg (CANA100)/MET, CANA 300 mg (CANA300)/MET, CANA100, CANA300, or MET. Primary end point was change in HbA1c at week 26 for combinations versus monotherapies. Secondary end points included noninferiority in HbA1c lowering with CANA monotherapy versus MET; changes in fasting plasma glucose, body weight, and blood pressure; and proportion of patients achieving HbA1c <7.0% (<53 mmol/mol). RESULTS From mean baseline HbA1c of 8.8% (73 mmol/mol), CANA100/MET and CANA300/ MET significantly lowered HbA1c versus MET (median dose, 2,000 mg/day) by –1.77%, –1.78%, and –1.30% (–19.3, –19.5, and –14.2 mmol/mol; differences of 1Dallas Diabetes and Endocrine Center at Medi- 2 – – – P cal City, Dallas, TX 0.46% and 0.48% [ 5.0 and 5.2 mmol/mol]; = 0.001) and versus CANA100 2 – – – – Diablo Clinical Research, Walnut Creek, CA and CANA300 by 1.37% and 1.42% ( 15.0 and 15.5 mmol/mol; differences of 3Institute of Experimental and Clinical Therapeu- –0.40% and –0.36% [–4.4 and –3.9 mmol/mol]; P = 0.001). -

Combination Use of Insulin and Incretins in Type 2 Diabetes
Canadian Agency for Agence canadienne Drugs and Technologies des médicaments et des in Health technologies de la santé CADTH Optimal Use Report Volume 3, Issue 1C Combination Use of Insulin and July 2013 Incretins in Type 2 Diabetes Supporting Informed Decisions This report is prepared by the Canadian Agency for Drugs and Technologies in Health (CADTH). The report contains a comprehensive review of the existing public literature, studies, materials, and other information and documentation (collectively the “source documentation”) available to CADTH at the time of report preparation. The information in this report is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. The information in this report should not be used as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process, nor is it intended to replace professional medical advice. While CADTH has taken care in the preparation of this document to ensure that its contents are accurate, complete, and up to date as of the date of publication, CADTH does not make any guarantee to that effect. CADTH is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in the source documentation. CADTH is not responsible for any errors or omissions or injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the information in this document or in any of the source documentation. -

ANTI-HYPERGLYCEMIC DIABETES AGENTS in T2DM: Color Outcomes Comparison Summary Table
ANTI-HYPERGLYCEMIC DIABETES AGENTS in T2DM: Outcomes Comparison Summary Table L Regier BSP BA, M LeBras PharmD, T Trischuk PharmD, J Bareham BSP, L Lu BSP © www.RxFiles.ca Aug 2021 Drug Class Sulfonylureas TZDs Meglitinides DPP4 Inhibitors GLP1 Agonists *** SGLT2 Inhibitors *** Insulin in T2DM Generic Metformin Gliclazide Glyburide Pioglitazone Rosiglitazone Acarbose Repaglinide Saxagliptin ONGLYZA Liraglutide VICTOZA Empagliflozin JARDIANCE Intensity: Intensity: BRAND (MF) DIAMICRON DIABETA ACTOS, g AVANDIA GLUCOBAY GLUCONORM Sitagliptin JANUVIA Exenatide BYETTA, BYDUREON Canagliflozin INVOKANA Less More GLUCOPHAGE Dulaglutide TRULICITY Dapagliflozin FORXIGA, FARXIGA Alogliptin NESINA (e.g. NPH (Multiple daily GLUCOTROL D/C STEGLATRO Glipizide Nateglinide Semaglutide OZEMPIC, RYBELSUS (PO) Ertugliflozin Linagliptin TRAJENTA HS + MF) doses) STARLIX D/C SPREAD-DIMCAD] Lixisenatide ADLYXINE; ALBIGLUTIDE D/C SAVOR-TIMI 53, EMPA-REG, CANVAS, CREDENCE, T2DM: UKPDS-33,80; ADVANCE, Major trials to UKPDS- ProACTIVE ACE 33,34,80 UKPDS- Meta-analysis. TECOS, EXAMINE LEADER, EXSCEL, FREEDOM CVO, DECLARE, VERTIS-CV (2020), ACCORD, VADT, ORIGIN, DEVOTE support ADVANCE (Prevention (ADOPT; 33,80 Ferwana M. Meta- RECORD interim, PROLOGUE, REWIND, SUSTAIN-6, PIONEER-6, DAPA-HF, DAPA-CKD (2020), T1DM: DCCT/EDIC findings/ analysis 2013. trial: Stop- - some use in (ADOPT) ADOPT, DREAM CARMELINA, EMPEROR-Reduced & -Preserved (Also Boussageon et al. Meta- Outcomes* ADVANCE) SR-Liao 2017; IRIS NIDDM) ELIXA, HARMONY CAROLINA (2020), EMPA-Kidney (2022) analysis. -

Dipeptidyl Peptidase-4 (DPP-4)
Pharmacy Medical Necessity Guidelines: Dipeptidyl Peptidase-4 (DPP-4) Inhibitors Effective: January 1, 2021 Prior Authorization Required √ Type of Review – Care Management Not Covered Type of Review – Clinical Review √ Pharmacy (RX) or Medical (MED) Benefit RX Department to Review RXUM These pharmacy medical necessity guidelines apply to the following: Fax Numbers: Commercial Products RXUM: 617.673.0988 Tufts Health Plan Commercial products – large group plans Tufts Health Plan Commercial products – small group and individual plans Tufts Health Freedom Plan products – large group plans Tufts Health Freedom Plan products – small group plans • CareLinkSM – Refer to CareLink Procedures, Services and Items Requiring Prior Authorization Tufts Health Public Plans Products Tufts Health Direct – A Massachusetts Qualified Health Plan (QHP) (a commercial product) Tufts Health Together – MassHealth MCO Plan and Accountable Care Partnership Plans Tufts Health RITogether – A Rhode Island Medicaid Plan Note: This guideline does not apply to Medicare Members (includes dual eligible Members). OVERVIEW FDA-APPROVED INDICATIONS The dipeptidyl peptidase-4 (DPP-4) inhibitors are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus as monotherapy or combination therapy. Per American Diabetes Association (ADA) guidelines, metformin and lifestyle changes are considered first line in the treatment of type 2 diabetes. DPP-4 inhibitors are considered add-on treatment options if glycemic targets are not achieved after approximately three months of metformin monotherapy. Sulfonylureas, thiazolidinediones, sodium-glucose co-transporter 2 (SGLT2) inhibitors, GLP-1 agonists, and basal insulin are also considered add-on treatments to metformin. The choice of add-on treatment is patient-specific. -

Medications Used to Treat Type 2 Diabetes
Medications Used to Treat Type 2 Diabetes This handout shows the different medications that your healthcare provider may prescribe to treat your type 2 diabetes, and where and how these medications work in your body to lower blood glucose. Type 2 diabetes medications are taken orally (by mouth), by injection (inserted into the fat under your skin), or inhaled (breathed in). Oral Injectable Alpha-glucosidase inhibitors (acarbose, miglitol) Amylin mimetic (pramlintide) Help to slow down the breakdown of starches (such Helps to decrease the amount of glucose made by your liver. as bread and potatoes) and certain types of sugar (such as table sugar) from your food in your intestines: Helps to slow down the breakdown of foods in your stomach this slows down increases in blood glucose. and intestines: this slows down increases in blood glucose Biguanide (metformin) GLP-1 receptor agonists (albiglutide, dulaglutide, exenatide, liraglutide) Helps to decrease the amount of glucose made by your liver Help your pancreas to make more insulin: insulin helps to lower blood glucose Helps to improve the way that insulin works in your Help to decrease the amount of glucose made by your muscles: if your muscles are more sensitive to insulin, it liver is easier for insulin to bring glucose from your blood into Helps to slow down the breakdown of foods in your muscles where glucose can be used for energy your stomach and intestines: this slows down increases in blood glucose DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin) Fat Tissue -

Diabetes: Putting It All Together to Design a High Value Diabetes Regimen for Your Patient
Diabetes: Putting it all together to design a high value diabetes regimen for your patient Cecilia C. Low Wang, MD Professor of Medicine Division of Endocrinology, Metabolism and Diabetes University of Colorado School of Medicine [email protected] Disclosures • None Objectives • Describe the benefits, side effects/risks, and costs of the newer diabetes medications • Discuss the value of the newer diabetes medications • Make high-value patient-centered decisions when intensifying diabetes therapy Worsening HbA1c • Ms L is a 48 year old woman here for routine f/u • She has Type 2 diabetes (x 5 years; no complications), obesity (BMI 41), chronic knee pain from osteoarthritis, and is postmenopausal s/p TAH 6 yr ago for uterine leiomyomas • You last saw her 6 months ago. At that time, her A1c was 6.4% on metformin 1000 mg BID • You see that her A1c is now 8.5%. • What other information do you need? • How would you intensify her diabetes treatment? “New-onset” diabetes • Mr. B is a 53 year old M hospitalized for NSTEMI and underwent 3v CABG • He had HTN but no other medical history and was on no medications prior to admission • Admission point-of-care (POC) glucose: 263 mg/dL • HbA1c: 11.5% • He agrees that he needs to be on insulin upon discharge • Are there any non-insulin medications that should be considered for him? • How would you design his regimen? ADVANCE: Diabetes complications and A1c relationship CVA CV death • Linear relationship (no threshold) for eye complications • J-curve: Increasing DKD Retinopathy risk with A1c <6.5% for CV and renal complications 6 Zoungas, et al. -

Glycemic Management of Type 2 Diabetes
Glycemic Management of Type 2 Diabetes Gail Nunlee-Bland, M.D. Professor Medicine & Pediatrics Director, Diabetes Treatment Center Howard University 1 Disclosures • None Learning Objectives • Understand the importance of lifestyle therapy in diabetes management • Know the classes of antihyperglycemic agents, mechanism of action, benefits and side effects of these agents • Recognize the importance of individualized treatment goals for diabetic patients AACE Comprehensive Care Plan Disease management from Antihyperglycemic a multidisciplinary team pharmacotherapy Comprehensive Care Plan Comprehensive diabetes Therapeutic lifestyle self-education for the change patient 4 Handelsman YH, et al. Endocr Pract. 2015;21(suppl 1):1-87. Glycemic Management of Type 2 Diabetes THERAPEUTIC LIFESTYLE CHANGE 5 Components of Therapeutic Lifestyle Change • Healthful eating • Sufficient physical activity • Sufficient sleep • Avoidance of tobacco products • Limited alcohol consumption • Stress reduction 6 Handelsman YH, et al. Endocr Pract. 2015;21(suppl 1):1-87. Glycemic Management of Type 2 Diabetes ANTIHYPERGLYCEMIC THERAPY 7 Cardiovascular Outcomes Trials: A Brief History • 2008 FDA guidance mandating assessment of CV safety of all antihyperglycemic agents in RCTs – Designed as noninferiority studies to demonstrate study drug was not associated with more MACE than placebo • Some study designs tested for superiority if noninferiority criteria were met – Primary endpoint: composite of cardiovascular death, nonfatal MI, and nonfatal stroke • Some primary